

Background: Ultrasound is an imaging technique widely used in patients with rheumatic and musculoskeletal diseases (RMDs) to detect signs of inflammation and structural damage. Factors such as nomenclature, definitions of ultrasound-detected pathologies, scoring systems and technical issues may affect the validity and generalisability of results of ultrasound studies in RMDs. These aspects, along with critical design characteristics, are often suboptimally reported in current ultrasound studies. A few years ago, a European Alliance of Associations for Rheumatology (EULAR) taskforce published a 23-item recommendation checklist to ensure transparent and comprehensive reporting of ultrasound research [1].
Objectives: To assess the implementation of the EULAR recommendation checklist since its development, and to compare ultrasound studies before and after the publication of the checklist.
Methods: Web of Science Core Collection und SCOPUS databases were screened by a medical information specialist for citations for the checklist. The identified articles were evaluated by two independent reviewers for appropriate study type (original research) and content (ultrasound). Based on the identified articles which cited the checklist, a second set of articles, published before the checklist became available, was generated. Articles in the second set were randomly selected from a list of articles matched for topic and journal to those in the first set. A pre-defined sheet containing 47 components based on the original checklist was used to extract data from both sets of articles (before and after). Paired Student´s T-test was utilized to compare the number of checklist components between the two groups of articles.
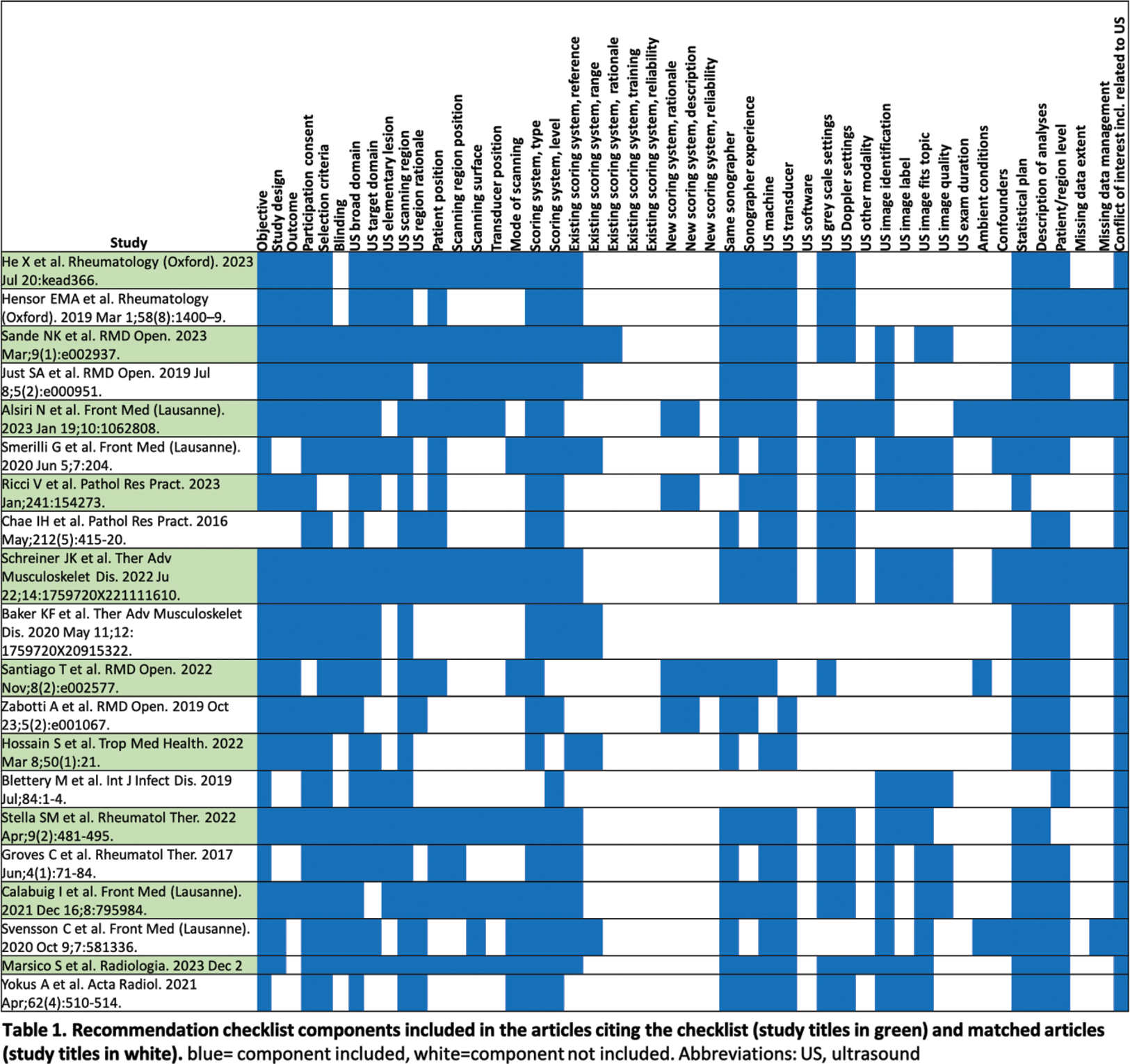
Results: A total of 24 articles cited the EULAR recommendation checklist. Of these 14 were excluded due to inappropriate study type (n:13 comment, viewpoint or review) or lack of use of ultrasound (n:1). The finally included 10 articles were matched to topic (10/10) and journal (8/10). Among the checklist components, those most frequently mentioned included ultrasound broad domain (100%), conflict of interest related to ultrasound (100%), objective (95%), participation consent (95%) and selection criteria (95%), ultrasound machine (85%) and ultrasound transducer (85%), while ultrasound software (0%), reliability of existing scoring system (0%), training of existing scoring system (0%), ultrasound exam duration (5%) or reliability of new scoring system (5%) were the least likely to be included in the articles (Table 1). The median number of checklist components included in the articles citing the checklist was significantly higher as compared to the matched articles published before the checklist was developed (median, interquartile range 31; 9.25 vs. 23; 7.25; p<0.001).
Conclusion: Our data suggests that the recently developed EULAR recommendation checklist facilitates the transparent and comprehensive reporting of ultrasound in studies utilising this imaging modality in RMDs.
REFERENCES: [1] Costantino F, Carmona L, Boers M, et al EULAR recommendations for the reporting of ultrasound studies in rheumatic and musculoskeletal diseases (RMDs). Ann Rheum Dis 2021;80:840-847.
Acknowledgements: NIL.
Disclosure of Interests: Peter Mandl AbbVie, Janssen, AbbVie, Novartis, Janssen, Enrico De Lorenzis: None declared, Federico Giuseppe Lazzaro: None declared, Brigitte Wildner: None declared, Maria Antonietta D’Agostino: None declared.