

Background: Drug survival of biologic and targeted synthetic disease-modifying antirheumatic drugs (ts/bDMARDs) has been described as a surrogate for treatment effectiveness and safety [1].
Objectives: To describe the drug survival of ts/bDMARDs in patients with Immune-mediated inflammatory arthritis (IIA) from five Latin American countries, using BIOBADA Registries data.
Methods: Data from BIOBADA Registries were collected from Argentina, Brazil, Mexico, Paraguay, and Uruguay (the last two countries were included in the same registry). For this analysis, we included all patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpa) who had started at least one biological or small molecule drug until October 2023. Biologic drug survival was defined as the time from initiation of therapy to discontinuation. The reasons for discontinuation were recorded. Drug survival was analysed using Kaplan-Meier plots, and hazard ratios were estimated.
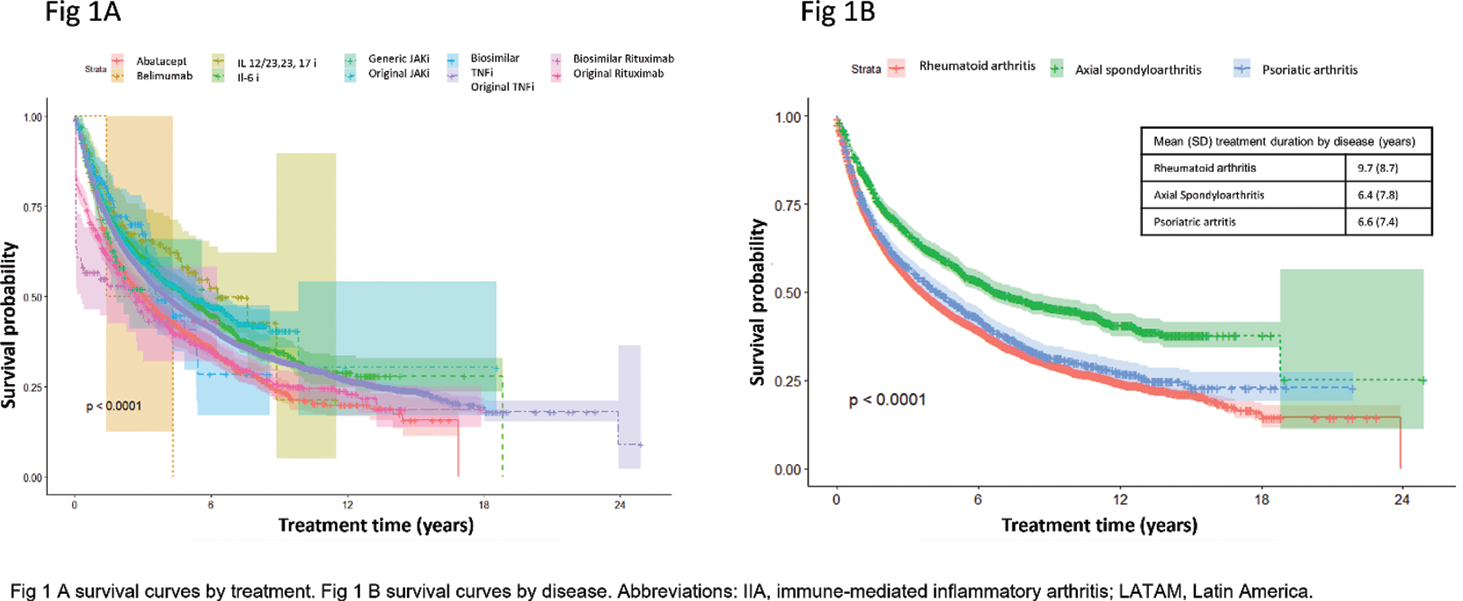
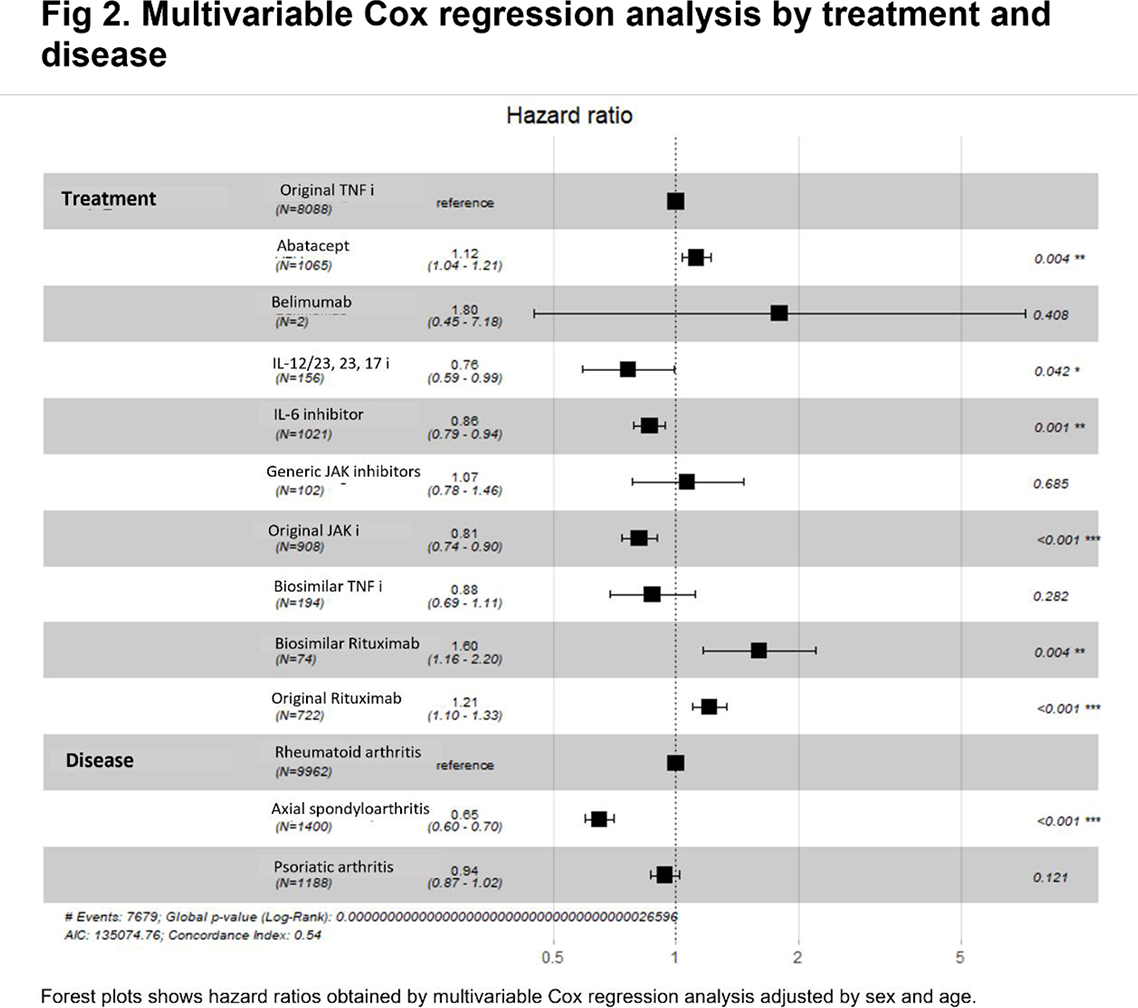
Results: Among 7098 registered patients, 12553 treatments were recorded, 5448 (43.4%) from Argentina, 4826 (38.4%) from Brazil, 1085 (8.6%) from Mexico, 706 (5.6%) from Paraguay and 488 (3.9%) from Uruguay. The most common diagnosis was RA with 9962 (79.3%) treatments, followed by 1400 (11.1%) with PsA and 1191 (9.6%) axSpa. A total of 7821 (62.3%) treatment discontinuations were reported. The overall mean treatment was was 8.9 (SD 8.6) years. The most common discontinuation causes were ineffectiveness (3056, 39.1%) and adverse events (1439, 18.4%). From the total of treatments from each bDMARDs, the most frequently discontinued were original Rituximab (RTXo) (489 of 722, 67.7%), abatacept (735 of 1065, 66.6%) and original TNF inhibitors (anti-TNFo) (5279 of 8088, 65.3%). From tsDMARDs, original JAK inhibitor was discontinued in 420 of 908 (46.3%) treatments and generic JAK inhibitor was in 39 of 102 (38.2%). Figure 1 A shows survival curves by treatment and Figure 1 B by disease. Figure 2 displays hazard ratios. Significant differences by treatment were reported with abatacept (HR 1.1, 95% CI 1.04-1.21, p=0.004), IL-12/23, 23 and 17 inhibitors (HR 0.76, 95% CI 0.59-0.99, p=0.001), original JAK inhibitors (HR 0.81, 95% CI 0.74-0.90, p<0.001), biosimilar rituximab (HR 1.60, 95% CI 1.16-2.20, p=0.004) and RTXo (HR 1.21, 95% CI 1.10-1.33, p<0.001. Significant differences by diagnoses were reported, axSpa (HR 0.65, 95% CI 0.60-0.70, p<0.001).
Conclusion: This analysis shows differences in the drug survival of ts/bDMARDs in Latin American patients with IIA by treatment and by diagnoses. Further longitudinal analyses will be performed to identify predictive variables.
REFERENCES: [1] Yiu ZZN, et al. Drug Survival Associated With Effectiveness and Safety of Treatment With Guselkumab, Ixekizumab, Secukinumab, Ustekinumab, and Adalimumab in Patients With Psoriasis. JAMA Dermatol. 2022;158(10):1131-1141.
Acknowledgements: NIL.
Disclosure of Interests: Deshire Alpizar-Rodriguez GSK Mexico, Carolina Ayelen Isnardi: None declared, Vijaya Rivera Terán: None declared, Ieda Maria Laurindo: None declared, Guillermo Pons-Estel: None declared, Maria Haye Salinas: None declared, Ida Elena Exeni: None declared, Rodolfo Nicolas Alvarado: None declared, Graciela Gomez: None declared, Alejandro Brigante: None declared, Marcelo Pinheiro: None declared, Roberto Ranza: None declared, Glaucio Castro: None declared, Vander Fernandes: None declared, Claiton Brenol: None declared, Marco Antonio Araujo da Rocha Loures: None declared, David Vega-Morales: None declared, Iris J. Colunga-Pedraza: None declared, Sandra Sicsik: None declared, Miguel A. Saavedra Salinas: None declared, Julio Cesar Casasola: None declared, Gabriela Avila: None declared, Sonia Cabrera-Villalba: None declared, Patricia Melgarejo: None declared, Paola Pusineri: None declared, Raquel Aranda: None declared, Paola Jara Gomez: None declared, Macarena Soto Estevez: None declared, Cristina Brunengo: None declared, Darwin Cordovilla: None declared, Belen Acevedo: None declared, Sandra Consani-Fernández: None declared, Sofia Rostan: None declared, Paloma de Abreu Trigueros: None declared.