

Background: Guselkumab (GUS), a fully human interleukin (IL)-23p19-subunit inhibitor, has demonstrated significant and durable efficacy and high rates of patient retention in randomized controlled trials of adults with active psoriatic arthritis (PsA). [1,2] A recent analysis of United States (US) health plan claims data showed persistence of on-label GUS (i.e., following US Food and Drug administration [FDA]-approved dosing of 100 mg administered by subcutaneous [SC] injection at Week [W] 0, W4, Q8W) at 12 months to be ~3x that of a first SC tumor necrosis factor inhibitor (TNFi). [3] Evidence in real-world adults with active PsA is also needed to compare on-label treatment persistence between patients who initiate GUS versus a first SC IL-17A inhibitor (IL-17Ai).
Objectives: To compare the on-label treatment persistence in patients with PsA receiving GUS versus SC IL-17Ai.
Methods: Biologic-naïve and biologic-experienced adults with active PsA, defined by ≥2 claims with a PsA diagnosis (International Classification of Diseases, 10 th Revision, Clinical Modification L40.5x) ≥30 days apart within 12 consecutive months before or on the index date (the first claim/start of treatment), and ≥1 claim for GUS or first SC IL-17Ai (ixekizumab or secukinumab) between 7/14/2020–6/30/2022 were identified in IQVIA PharMetrics® Plus. Selected patients also had ≥12 months of continuous enrollment and no claim for a potentially confounding rheumatic disease within 12 months preceding the index date (Figure 1). Baseline patient characteristics were balanced between the GUS and SC IL-17Ai cohorts using propensity score (standardized mortality ratio) weighting. On-label persistence (absence of discontinuation or dose escalation/reduction relative to FDA prescribing information) was described and compared using Kaplan-Meier survival analysis and Cox proportional hazard models in the weighted treatment cohorts.
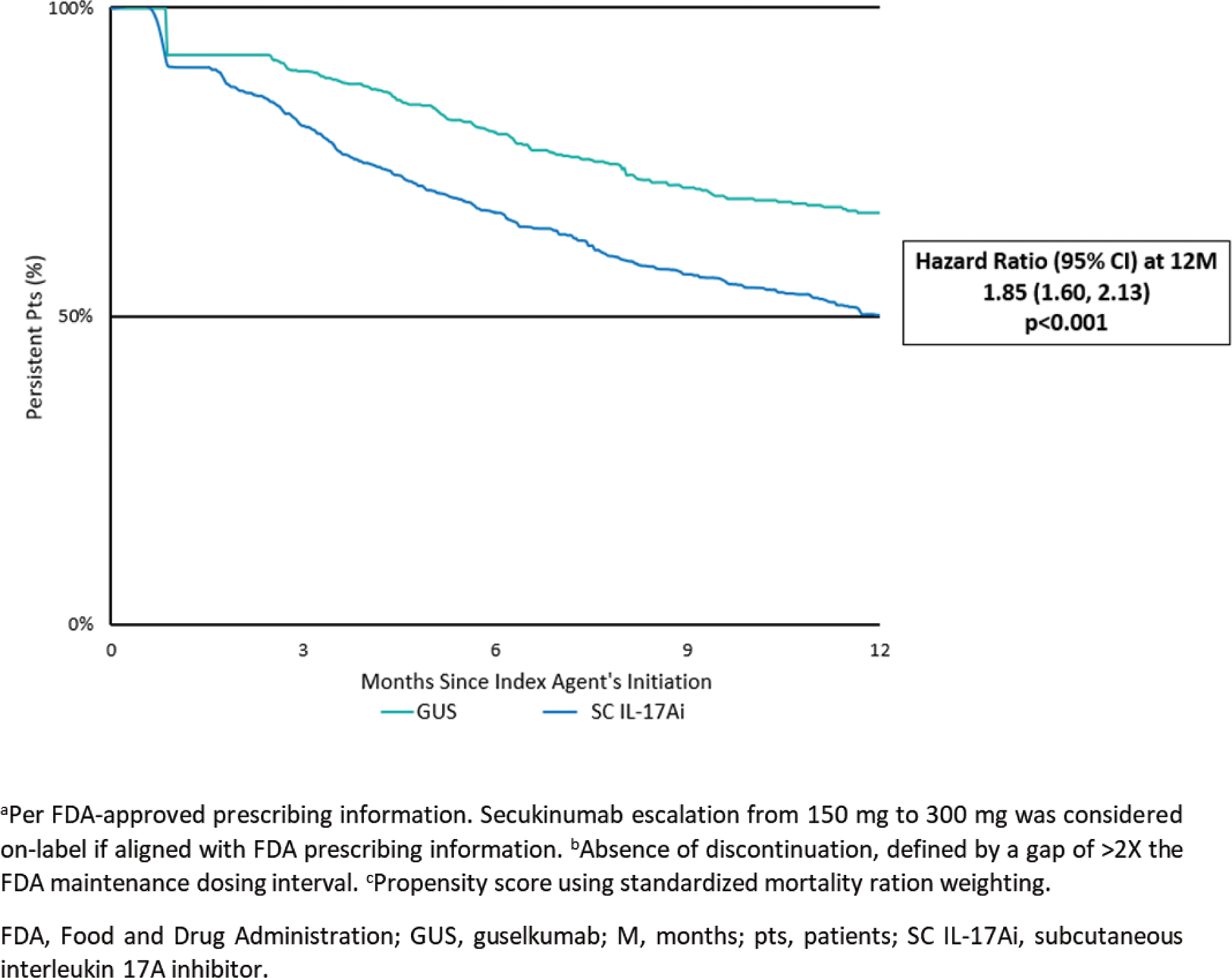
Results: The GUS and SC IL-17Ai cohorts included 910 (mean age 50 years, 60% female) and 2,743 (50 years, 58% female) patients, respectively. After weighting, baseline characteristics were well-balanced, with mean follow-up of 13.5–13.7 months and 52% of patients across cohorts having received biologics in the previous 12 months. Respective rates of treatment persistence at 3, 6, 9, and 12 months were 90%, 80%, 71%, and 67% for GUS versus 81%, 67%, 57%, and 50% for SC IL-17Ai (overall log-rank p<0.001). Patients in the GUS cohort were significantly more likely than those in the SC IL-17Ai cohort to remain persistent on treatment at each timepoint assessed, with increasing hazard ratios (HR) from 3 to 12 months (HR=1.85; p<0.001; Figure 2). Median time to discontinuation was not reached for GUS and was 12.3 months for SC IL-17Ai.
Conclusion: In this real-world study employing primarily commercial US health plan claims data to assess on-label treatment persistence in patients with PsA, GUS was associated with a significantly greater (nearly 2x) likelihood of persistence at 12 months compared with an initial SC IL-17Ai.
REFERENCES: [1] Ritchlin CT. RMD Open. 2021;7:e001457.
[2] McInnes IB. Arthritis Rheumatol. 2022;74:475-85.
[3] Walsh J. Comparison of On-label Treatment Persistence in Real-world Patents With PsA Receiving Guselkumab Versus Subcutaneous TNF Inhibitors. Poster presented at: CCR-W; 2023; San Diego, CA.
Data source and study design
Kaplan-Meier analysis of on-labela persistenceb in propesnity score weightedc GUS and SC IL-17Ai cohorts
Acknowledgements: NIL.
Disclosure of Interests: Natalie J. Shiff Owns or has owned stock in AbbVie, Gilead, Iovance, Johnson & Johnson, Novo-Nordisk, and Pfizer within the past 3 years. [Current stock ownership: AbbVie, Gilead, Iovance, Jazz, Johnson & Johnson, Novavax, and Viatris], Employee of Janssen Scientific Affairs, LLC; received salary support from the Childhood Arthritis and Rheumatology Research Alliance within the past 3 years, Philip J. Mease Speaker fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Consulting fees from AbbVie, Acelyrin, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Immagene, Janssen, Novartis, Pfizer, SUN, UCB, and Ventyx, Research grants from AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, SUN, and UCB, Ruizhi Zhao Owns stock in Johnson & Johnson, Former employee of Janssen Scientific Affairs, LLC, Shannon Ferrante Owns stock in Johnson & Johnson, Employee of Janssen Scientific Affairs, LLC, Soumya D. Chakravarty Owns stock in Johnson & Johnson, Employee of Janssen Scientific Affairs, LLC, Jessica A. Walsh Consulting for AbbVie, Eli Lilly, Janssen, Novartis, and UCB, Research funding from AbbVie, Merck, and Pfizer