

Background: In the pathogenesis of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), neutrophils play a crucial role, serving as both the target cells attacked by ANCA and the main participating cells in regulating the inflammatory process. [1]
Autoimmune and chronic inflammatory diseases can lead to abnormalities in leukocytes, particularly in polymorphonuclear cells (PMN), with neutrophils comprising the majority. Abnormal PMN morphologies may encompass hypo- and hyper-segmentation as well as immature forms. [2] There is a gap in the current literature regarding abnormalities in circulating leukocytes in patients in sustained remission.
Objectives: The aim of this study is to investigate the different neutrophil phenotypes within three groups: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA).
Methods: We collected blood samples from adult patients affected by GPA, MPA and EGPA, classified according to the EULAR/ACR criteria 2022, who attended the outpatients Vasculitis Clinic of our University. All patients were no longer taking steroid therapy and were in the remission stage, as per the EULAR/ACR definition. A age- and sex- matched control group of healthy subjects was enrolled. May-Graunwald-Giemsa (MGG) staining of peripheral blood smears was used for studying cellular morphology.
Results: Between may 2023 and November 2023, we enrolled 45 patients: 16 GPA patients, 13 MPA patients and 28 EGPA patients, of which 19 ANCA positive EGPA and 9 ANCA negative EGPA. Hence, we enrolled 28 matched healthy controls (HCs).
No differences were observed among the three groups of AAV patients with respect to female sex (GPA=50%, MPA=46.2%, EGPA=46.4%, p=0.969), age at sampling (median and IQR, GPA=54 [40-67], MPA=67 [52-78], EGPA=62 [55-71], p=0.172), disease duration at sampling (median and IQR, GPA=58 [43-143], MPA=90 [61-108], EGPA=78 [36-124], p=0.818), time from steroid discontinuation (median and IQR, GPA=36 [6-52], MPA=38 [14-53], EGPA=18 [9-48], p=0.567), and ongoing immunosuppressive therapy at sampling (n and %, GPA=12 [75], MPA=9 [69], EGPA=18 [64], p=0.7611).
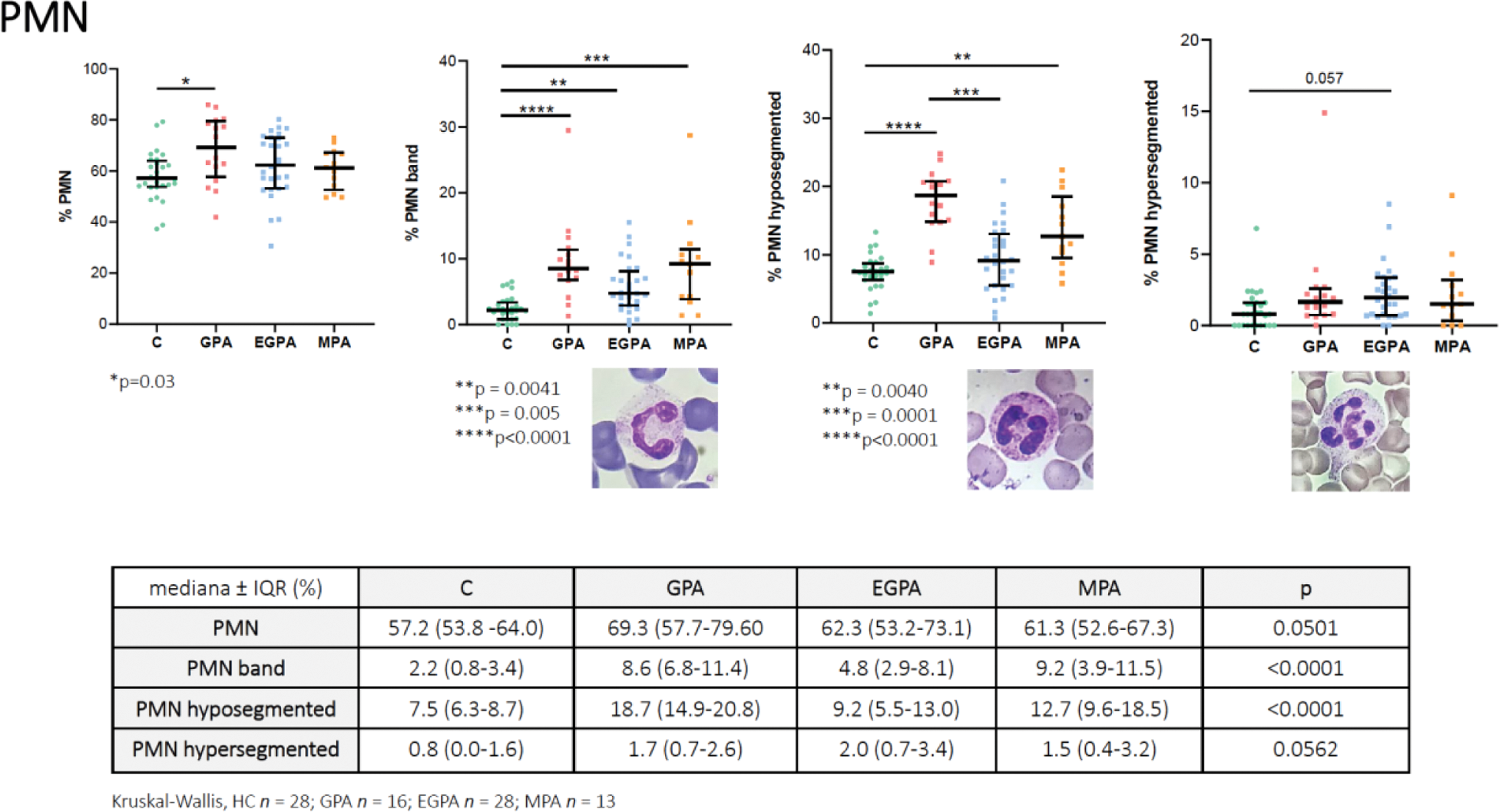
A statistically significant increase in total PMN numbers was observed in AAV when compared to HC (median and IQR, HC 57.2 [53.8 -64.0], GPA 69.3 [57.7-79.60}, MPA 61.3 [52.6-67.3], and EGPA 62.3 [53.2-73.1], p=0.0501).
Hence, there was a statistically significant increase in the number of hypo-segmented in AAV patients (PMN: HC 7.5 [6.3-8.7], GPA 18.7 [14.9-20.8], MPA 12.7 [9.6-18.5], and EGPA 9.2 [5.5-13.0], p<0.0001), with a significant between the GPA and EGPA groups (p = 0.0001).
The number of hyper-segmented PMNs was similar in the four groups. No differences were observed in monocytes, granulocytes, and lymphocytes. Finally, no differences were observed in EGPA ANCA-positive patients when compared to EGPA ANCA-negative patients concerning PMN subgroups.
Conclusion: Patients with AAV exhibit statistically significant increase in the number of PMNs, particularly in the count of hypo-segmented PMNs. Further prospective studies involving larger cohorts are needed to confirm these differences and explore whether distinctions exist between active and inactive patients.
REFERENCES: [1] Ge S et al. Front Pharmacol . 2022;13:957660.
[2] Baggio C et al. Int J Mol Sci . 2023;24(6):5450.
Acknowledgements: NIL.
Disclosure of Interests: None declared.