

Background: Neurological involvement in rheumatoid arthritis (RA) is understudied despite a high prevalence of depression, cognitive deficits, chronic pain, and fatigue reported in RA patients. Furthermore, there is increasing interest in the role of peripheral and brain-resident innate immune cells in aging and neurodegenerative disease. Systemic immune activation has been shown to activate microglia and affect brain region size in models of RA [1,2], but how chronic inflammation affects the aging human brain has not been previously studied.
Objectives: We investigate regional brain volumes in RA patients compared to healthy age- and sex-matched controls and relate differences to neuropsychiatric symptoms and peripheral blood CD14+ monocyte phenotypes.
Methods: We included 71 female patients (median age 64 years, range 23-76) with established RA (disease duration 10 years, range 0-45) and 268 healthy women (median age of 54 years (21-82)) who served as controls (C). T1-weighted cranial magnetic resonance images were used to measure 120 brain regions. For analysis of brain region differences in relation to age, RA patients and controls were binned into age intervals of 5 years: 0-45y (RA: n=7, C: n=98), 45-50y (RA: n=4, C: n=15), 50-55y (RA: n=11, C: n=26), 55-60y (RA: n=7, C: n=32), 60-65y (RA: n=13, C: n=41), 65-70y (RA: n=21, C: n=25), older than 70y (RA: n=8, C: n=31). Patient-reported neuropsychiatric symptoms were assessed with the Fibromyalgia Impact Questionnaire (FIQ). Peripheral blood CD14+ monocytes were isolated and analysed with RNA sequencing. Genes and pathways associated with lateral ventricle size was identified by comparing patients with lateral ventricle size smaller and larger than 30 cm 3 .
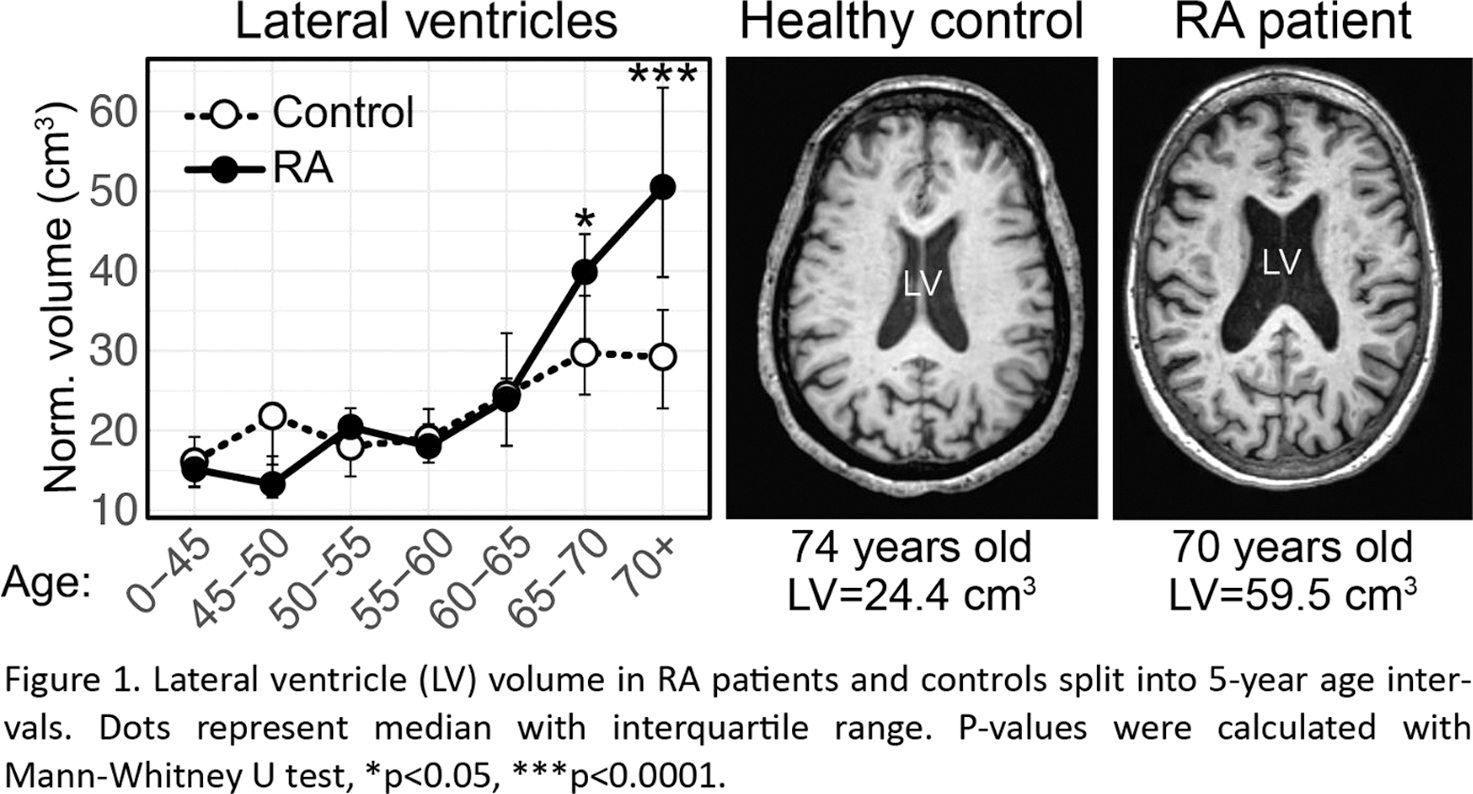
Results: RA patients over 65 had significantly enlarged lateral ventricles of the brain compared to healthy controls of the same age range (Figure 1). While there were no differences in lateral ventricle size between RA patients and controls in participants under 65 years, the lateral ventricles were enlarged by 25% (p=0.020) in patients 65-70, and 42% (p=0.00020) in patients over 70. Next, we analysed regional brain volumes in the 71 RA patients compared to 71 age-matched controls. We found that 7 limbic and 5 cortical regions were significantly different in both the left and right hemisphere. Of these regions, the thalamus, the middle frontal gyrus and the superior frontal gyrus had strong inverse correlations to the lateral ventricle volume (thalamus: Spearman r=-0.46, p=0.0002; middle frontal gyrus: r=-0.56, p<0.0001; superior frontal gyrus: r=-0.36, p=0.0052). Interestingly, the reduced thalamus size was associated with depression reported in the FIQ questionnaire according to a linear model controlling for the patients age (Beta=-21, p=0.0080).
Monocytes share characteristics with microglia and can migrate to the brain. We found that CD14+ monocytes from patients with larger lateral ventricles had several activated pathways related to neuroinflammation, including neuroinflammation signalling and multiple-sclerosis signalling. Upregulated genes included CYBB, MSR1, VEGFA, HLA-DRA, A2M and CD9, which implies neurodegenerative microglia phenotypes [3].
Conclusion: RA patients over the age of 65 had larger lateral cerebral ventricles, indicating brain atrophy possibly caused by accelerated aging or neurodegeneration. Enlarged ventricles were likely a consequence of shrinkage of the thalamus as well as frontal cortical regions and were associated with neuropsychiatric symptoms in RA patients. Monocytes of patients with enlarged lateral ventricles had a phenotype associated with neuroinflammation and disease-associated microglia. A limitation to our study is an insufficient sample size for rigorous statistical testing using a split-sample approach. We therefore present our generated hypotheses along with statistical test results as suggestions for future rigorous testing on independent data.
REFERENCES: [1] Anderson & Wasén et al., PNAS 2019
[2] Süß et al., Cell Reports 2020
[3] Butovsky & Weiner, Nature Reviews Neuroscience 2018
Acknowledgements: NIL.
Disclosure of Interests: None declared.