

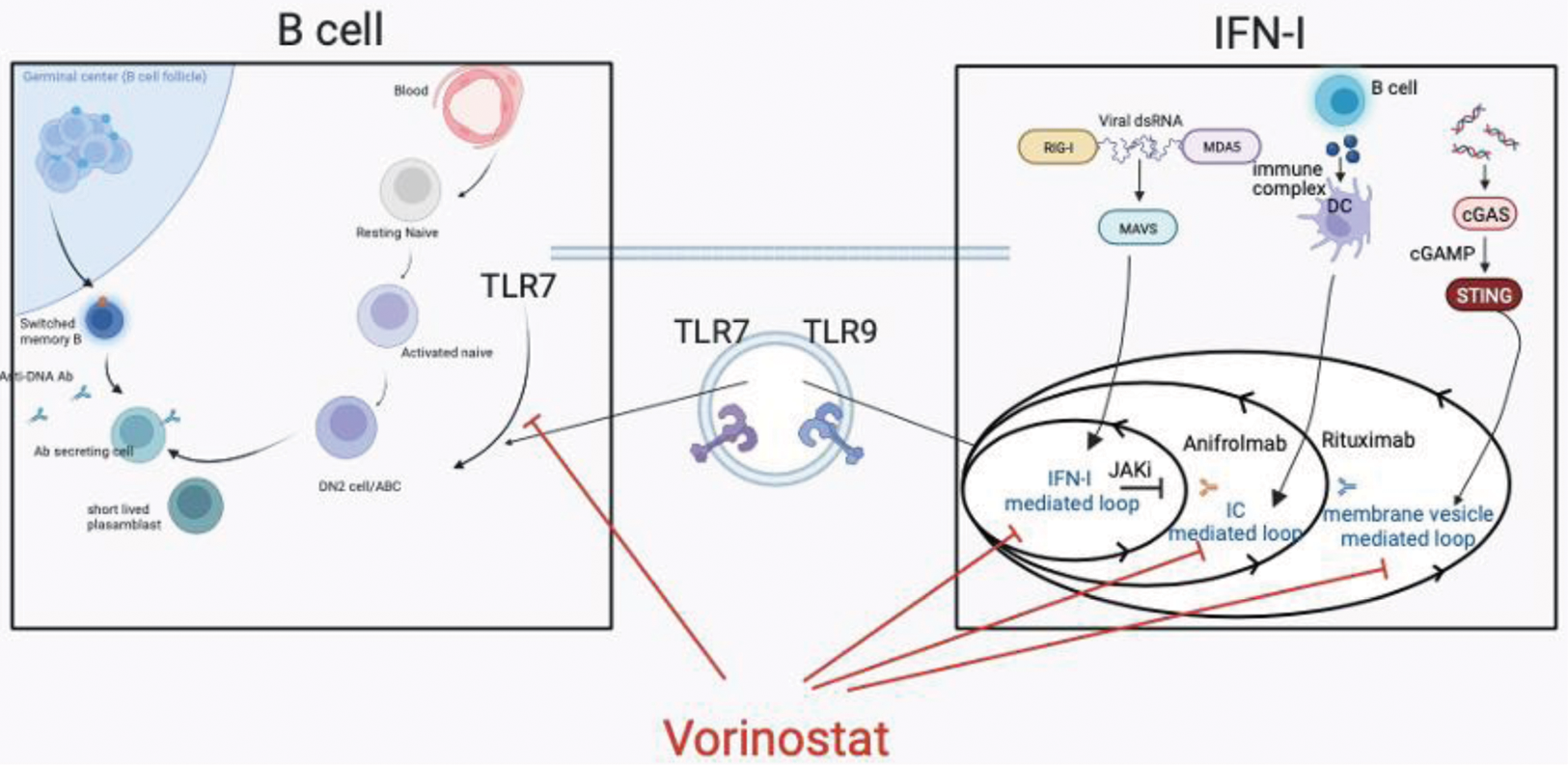
Background: SLE is a systemic autoimmune disease caused by impaired innate and acquired immune tolerance, resulting in increased Type I IFN (IFN-I) and aberrant B cell development. Upon sensing nucleic acid, IFN-I is produced via multiple pathways, including RIG-I-like receptors-, Toll-like receptors- (TLR), and cGAS-mediated pathway, In particular, TLR7 stimulation lead to autoantibody production via promoting extrafollicular differentiation B cell into the pathological B cell populations, such as active naive B cells, double negative B cells, and short-lived plasmablasts. Increased IFN-I and pathogenic B cell activity are synergistically involved in disease activity at onset and relapse. However, there have been no therapeutic agents that simultaneously inhibit both IFN-I production and abnormal B cell maturation.
Objectives: We aimed to identify inhibitors from among clinically approved drugs that have the potential to inhibit both IFN-I production and abnormal B cell maturation. We then aimed to elucidate the molecular mechanisms by which they inhibit both IFN-I production and abnormal B cell maturation, and their therapeutic efficacy in mouse models.
Methods: We screened the candidate compounds using the reporter cell that can monitor IFN-I induction and chemical library consisting of clinically approved drugs. We then examined whether they suppress the expression and phosphorylation of upstream signaling molecules for IFN-I and the differentiation of B cells into plasma cells, and whether they can alleviate disease severity in SAVI mice and NZB/W F1 mice.
Results: We identified that Vorinostat, a clinically approved pan-HDAC inhibitor, inhibited both IFN-I production and B cell differentiation. Vorinostat inhibited TBK1 phosphorylation and following IRF3 nuclear translocation, and suppressed the expression of both IFN-I–inducing molecules, such as IRF5, and IRF7, and B cell-related genes, such as VpreB. Additionally, Vorinostat suppressed lung inflammation and fibrosis in SAVI mice due to suppressing IFN-I and alleviated the mortality and renal disease of NZB/W F1 mice by suppressing IFN-I and B cells differentiation. Furthermore, Vorinostat suppressed TLR7-mediated plasma cell differentiation from human B cells.
Conclusion: Vorinostat has a potential to simultaneously inhibit IFN-I production and aberrant B cell development by suppressing TLR7-mediated signaling. Vorinostat would have the advantage of being in clinical use and can be rapidly delivered to SLE patients as a novel agent that selectively affects both IFN-I and B cells.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.