

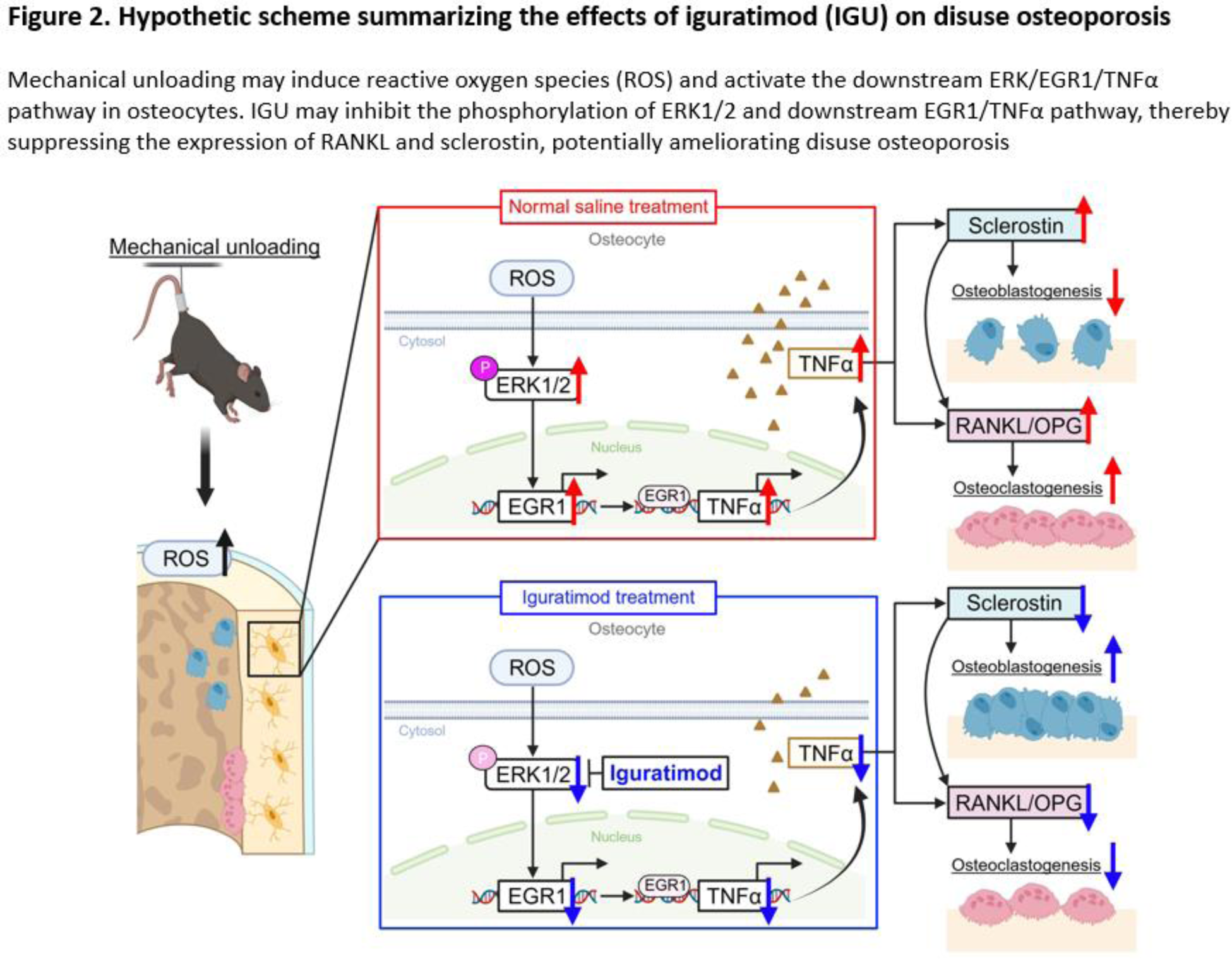
Background: Disuse osteoporosis is a prevalent complication among patients with rheumatoid arthritis (RA). The treatment of disuse osteoporosis has been reported to diminish the efficacy of conventional osteoporosis drugs. Therefore, alternative therapeutic approaches distinct from conventional osteoporosis treatments are necessary. A meta-analysis has reported that iguratimod (IGU), a small molecule disease-modifying antirheumatic drug, prevented bone loss and improved bone metabolisms in patients with RA [1]. However, details regarding its effects on disuse osteoporosis and osteocytes remain unclear. In disuse osteoporosis, upregulation of reactive oxygen species (ROS), followed by phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and consequent upregulation of early growth response protein 1 (EGR1) and tumor necrosis factor alpha (TNFα) leads to increased production of receptor activator of NF-κB ligand (RANKL) and sclerostin from osteocytes. RANKL induces osteoclastogenesis, while sclerostin inhibits osteoblastogenesis, which mechanisms induce systemic bone loss.
Objectives: The current study examined the effects of IGU on osteocytes using a mouse model of disuse-induced osteoporosis, the pathology of which crucially involves osteocytes.
Methods: Eight-week-old C57BL/6J male mice underwent 21 days of hindlimb unloading (HLU) by tail suspension, and 200 μl of normal saline (NS) or IGU (30 mg/kg) was intraperitoneally injected 5 times per week over the same period. Mice were randomly divided into three groups (n=8 in each group): normal mice with NS (NS group), HLU with NS (HLU + NS group), and HLU with IGU (HLU + IGU group). Micro-computed tomography (μCT) was utilized to analyze the trabecular and cortical bone of the distal femurs. Bone histomorphological features were assessed by tartrate-resistant acid phosphatase (TRAP) staining and immunostaining of osteocalcin, sclerostin, ROS, EGR1, and TNFα. Additionally, the effect of IGU on bone metabolism were investigated in vitro. The NF-κB inhibitory effect of IGU was evaluated by luciferase assay and immunofluorescence staining in osteocytes. The relationship between disuse osteoporosis and the effects of IGU on osteocytes was analyzed by RNA sequencing. The EGR1 overexpression vector pCMV6-EGR1, and EGR1 small interfering RNA (siRNA) were used to assess the role of EGR1 in osteocytes.
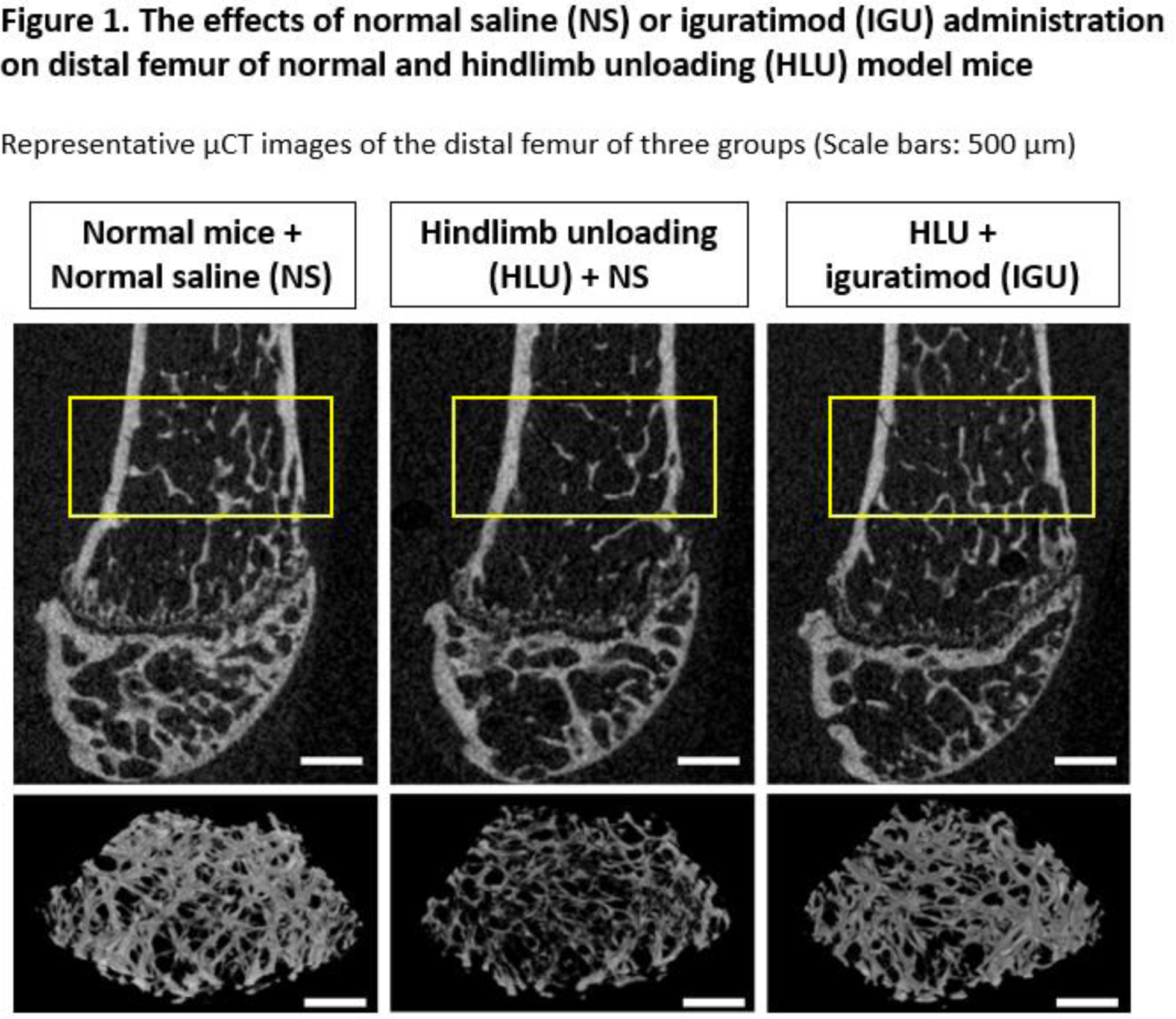
Results: A reduction in distal femur bone mass was achieved after HLU in mice, which was subsequently reversed by intraperitoneal IGU treatment (Figure 1). Histology revealed that HLU mice had significantly increased osteoclast number and sclerostin-positive osteocyte rates, which were significantly suppressed by IGU treatment. Moreover, HLU mice exhibited a significant decrease in osteocalcin-positive cells, which was attenuated by IGU treatment. In vitro, IGU suppressed the gene expression of RANKL and sclerostin in MLO-Y4 and Saos-2 cells, which inhibited osteoclast differentiation of mouse bone marrow cells in cocultures. Although IGU did not affect the nuclear translocation or transcriptional activity of NF-κB and production of ROS, RNA sequencing revealed that IGU downregulated the expression of mechanical stress-related factor, EGR1 in osteocytes. HLU mice showed significantly increased EGR1- and TNFα-positive osteocyte rates, which was decreased by IGU treatment. In vitro, IGU inhibited ERK1/2 phosphorylation, and EGR1 overexpression enhanced the gene expression of TNFα, RANKL, and sclerostin, which was suppressed by IGU in osteocytes. Contrarily, small interfering RNA-mediated suppression of EGR1 downregulated RANKL and sclerostin gene expression.
Conclusion: Our findings demonstrated that IGU inhibited sclerostin and RANKL production through the ERK/EGR1/TNFα pathway in osteocytes (Figure 2). These results indicate that IGU may have a potential for becoming a unique and effective treatment option for disuse osteoporosis by targeting osteocytes.
REFERENCES: [1] Li Deng, et al. Influence of Iguratimod on Bone Metabolism in Patients with Rheumatoid Arthritis: A Meta-analysis. Int J Clin Pract. 2022;28:1-14.
Acknowledgements: NIL.
Disclosure of Interests: Taihei Miura: None declared, Kosuke Ebina K. Ebina has received research grants and lecture fees from Eisai Co., Ltd., K. Ebina was affiliated with the Department of Musculoskeletal Regenerative Medicine, Osaka University Graduate School of Medicine, which is supported by Taisho Pharmaceutical Co., Ltd., The iguratimod was kindly provided by Toyama Chemical Co., Ltd (Tokyo, Japan)., Yuki Etani Y. Etani is affiliated with the Department of Musculoskeletal Regenerative Medicine, Osaka University Graduate School of Medicine, which is supported by Taisho Pharmaceutical Co., Ltd., Takaaki Noguchi: None declared, Makoto Hirao: None declared, Kenji Takami: None declared, Atsushi Goshima: None declared, Takuya Kurihara: None declared, Yuji Fukuda: None declared, Nagahiro Ochiai N. Ochiai is affiliated with the Department of Musculoskeletal Regenerative Medicine, Osaka University Graduate School of Medicine, which is supported by Taisho Pharmaceutical Co., Ltd. N. Ochiai is an employee of Taisho Pharmaceutical Co., Ltd., Takashi Kanamoto: None declared, Ken Nakata: None declared, Seiji Okada: None declared