

Background: Type I interferon plays a critical role in the pathogenesis of systemic lupus erythematosus (SLE), and the expression of type I interferon-stimulated genes (ISG) is generally up-regulated in SLE [1]. Type I ISG signature refers to the selected genes that represent the pattern of expression of type I ISG, and it is a widely used indicator in clinical trials for SLE, such as comparing the effectiveness of drugs by dividing patients into high and low type I ISG signature scores [2].
On the other hand, there is no uniform definition of type I ISG signature yet. They are usually determined by researchers or by citing past definitions in each report, and even the number of genes used is not standardized. This point is a major obstacle to using type I ISG signatures in correlation analysis with the clinical information of SLE. It is necessary to compare and evaluate multiple type I ISG signatures.
Objectives: The aim of this study is pursuing the best type I ISG signature suitable for the clinical evaluation of SLE activity. First, we calculated previously reported different kinds of type I ISG signature scores of RNA sequencing data obtained from SLE patients, and analyzed and compared the relationships with clinical information. Next, we calculated a comprehensive type I ISG signature scores produced by randomly combined 21 type I ISGs and attempted to extract characteristics of type I ISG signature suitable for evaluating SLE activity.
Methods: 120 peripheral blood samples were collected from 59 SLE patients recruited. For the 23 patients, samples were taken before treatment and several times after treatment. 14 patients were in LLDAS, 22 patients in DORIS remission. 20 samples were collected from 20 healthy subjects. RNA sequencing was performed with NovaSeq 6000 (Illumina) with a pair-end read of 100 base pairs. FASTQ format data were mapped with UCSC human genome 38 as the reference sequence.
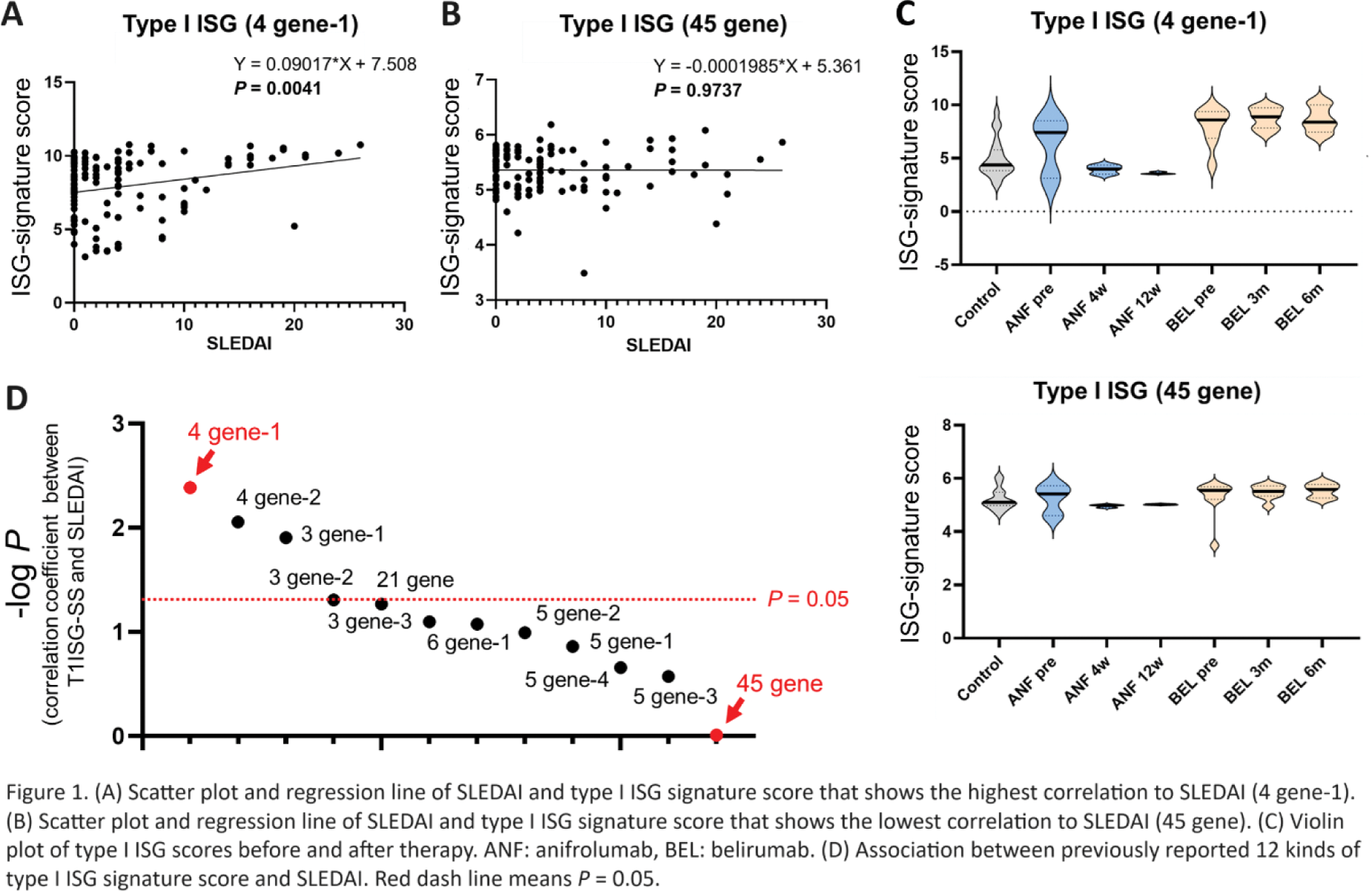
After standardization of raw expression data, previously reported 12 kinds of type I ISG signature scores were calculated. Correlation between type I ISG signature score and SLEDAI was evaluated by Pearson’s correlation coefficient.
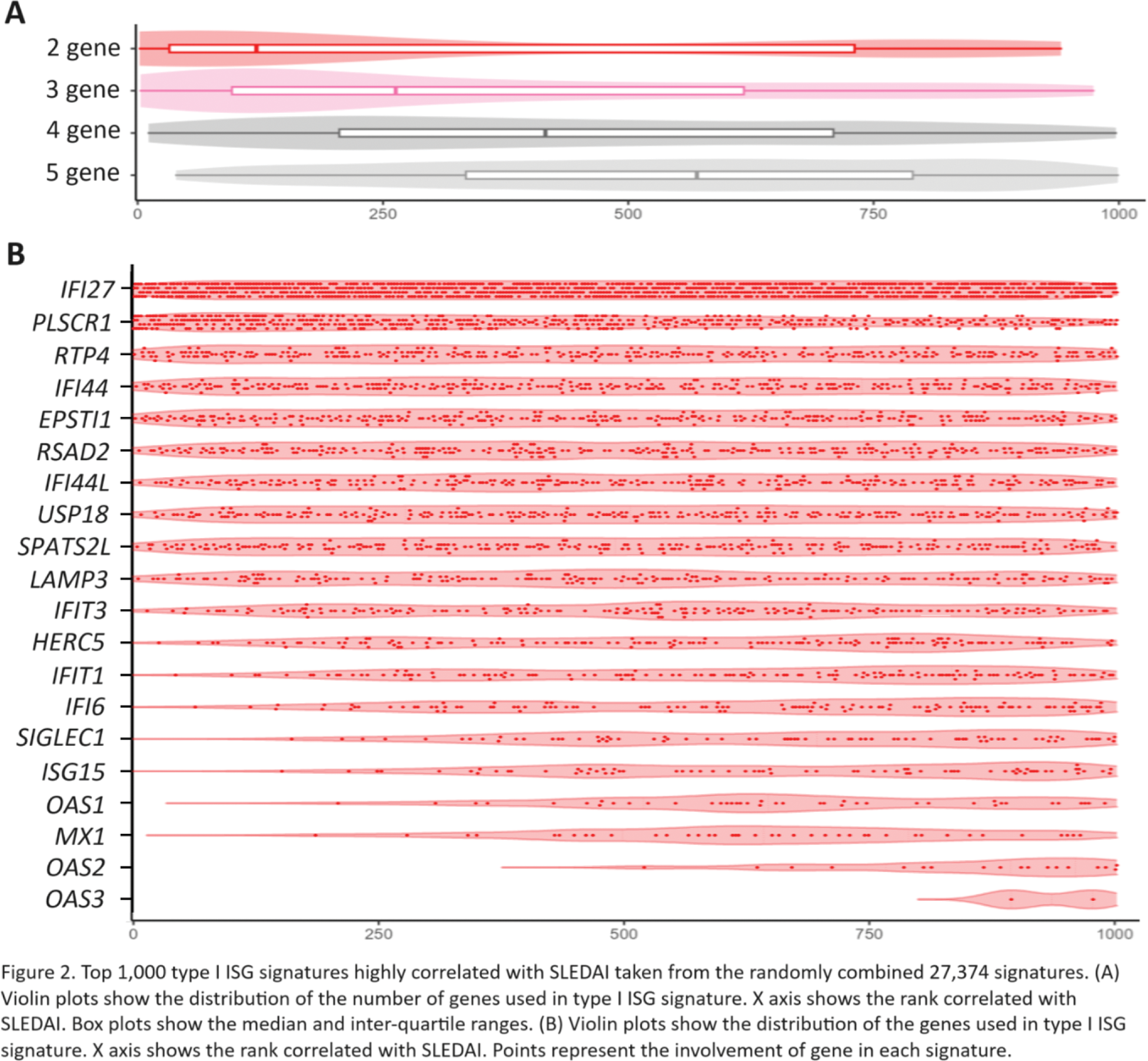
We then randomly combined two, three, four and five genes from representative 21 type I ISGs and produced total 27,374 type I ISG signatures. Correlation between each ISG signature score and SLEDAI was evaluated by Pearson’s correlation coefficient, and the character of type I ISGs signatures highly associated with SLEDAI were investigated.
Results: The correlation between the previously reported 12 type I ISG signatures and SLEDAI widely ranged from significant (r = 0.2647, P = 0.0041) (Figure 1A) to no correlation (r = -0.003091, P = 0.9737) (Figure 1B). The best correlated type I ISG signature score decreased over time after administration of anifrolumab, although the least correlated type I ISG signature score did not change (Figure 1C). Using fewer genes (three or four) had better correlation with SLEDAI compared with more genes (five and more) (Figure 1D).
Among top 1,000 type I ISG signatures highly correlated with SLEDAI taken from the randomly combined 27,374 signatures, there was a tendency for the smaller the number of selected genes, the higher the correlation with SLEDAI (Figure 2A). The best correlated type I ISG signature (r = 0.3136, P = 0.0006093) consisted of IFI27 and PLSCR1 . IFI27 and PLSCR1 were mainly included in the top type I ISG signature highly correlated with SLEDAI (Figure 2B).
Conclusion: When evaluating SLE activity using type I ISG signatures, the number of genes and the selected genes greatly affected the correlation of signature scores with SLEDAI and post-treatment changes. Two or three genes including IFI27 are important for evaluating SLE activity.
REFERENCES: [1] Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565-579.
[2] Furie R, et al. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69(2):376-386.
Acknowledgements: NIL.
Disclosure of Interests: Shuji Sumitomo Asahi-kasei, GSK, AstaraZeneca, Abbvie, Pfizer, Eli Lilly, Chugai, Astellas, BMS, Eizai, Mitsubishi-Tanabe, Hideki Oka: None declared, Hayato Shimizu: None declared, Takeshi Iwasaki: None declared, Maki Kanamori: None declared, Hiroaki Nishioka: None declared, Koichiro Ohmura Eisai, Gilead Sciences, AstraZeneca, GSK, Asahi-Kasei Pharma, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma.