

Background: VEXAS is a hemato-inflammatory syndrome that occurs in older males with an acquired somatic mutation in the UBA1 gene in myeloid lineage cells [1]. The most common variant is usually p.M41T, while p.M41V and p.M41L are less prevalent [1,2]. Several reports have found evidence for a genotype-phenotype interaction pointing to a worse prognosis in p.M41V pathogenic variant carriers [2,3].
Objectives: To characterize the epidemiology of VEXAS in Israel.
Methods: This is a descriptive cross-sectional multicenter national study done in January 2024 in Israel. We gathered the results of UBA1 gene testing for the diagnosis of VEXAS from genetic centers performing this test in Israel. We reached out to the medical centers treating VEXAS patients and collected anonymized data including demographic and clinical data, laboratory tests, treatments, and response to therapy. Categorical variables are presented as number (percent) and continuous variables are presented as mean±standard deviation and (range).
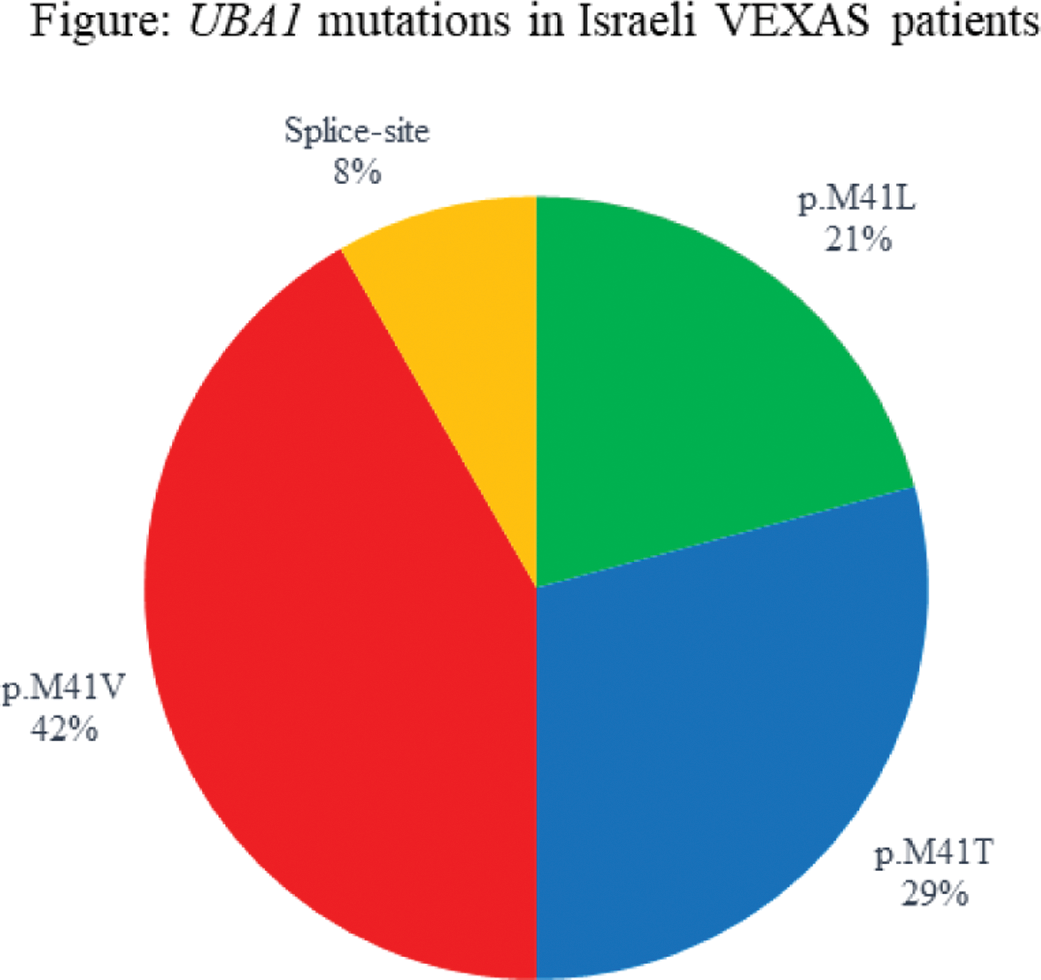
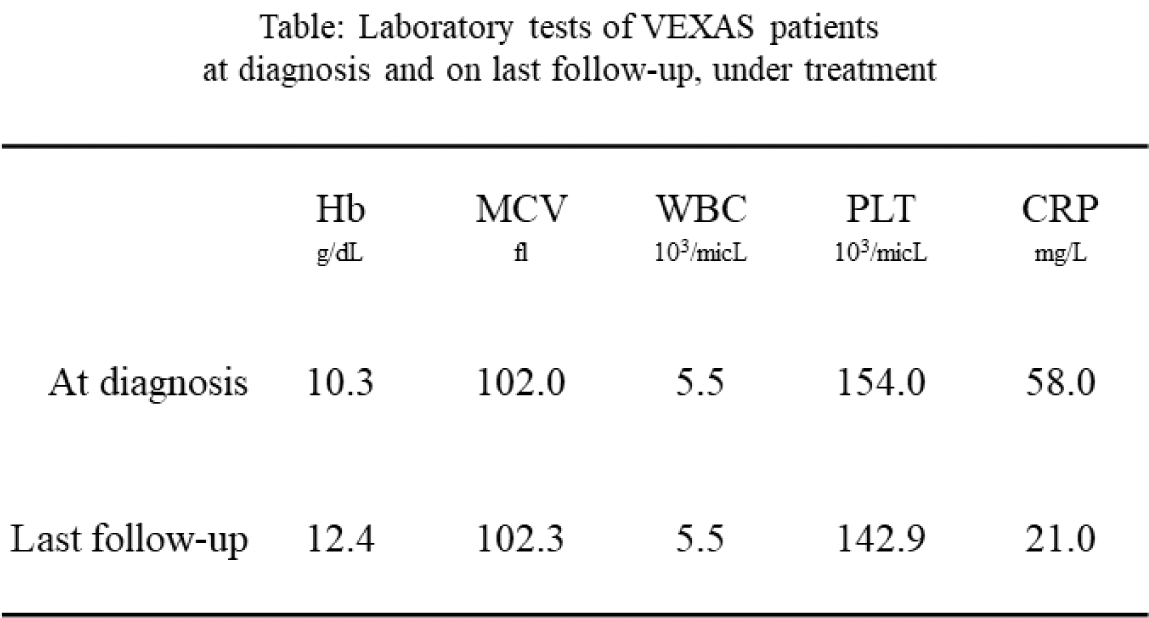
Results: Altogether 24 VEXAS patients were included, with a mean follow-up time from diagnosis of 1.8±1 (0-4) years. All patients were Jewish and male, age at diagnosis was 76.0±6.8 (56-88) years. Disease duration at the time of diagnosis was 1.5±1.5 years. The most prevalent pathogenic variant was the p.M41V (n=10, 42%) (Figure 2). Of 10 patients carrying the p.M41V mutation, 3 died (30%), as well as a 4 th patient with a splice-site mutation. Time from 1 st symptom to death was 1-3 years for the patients with p.M41V and 5 years for the splice-site patient. The mortality rate in the Israeli cohort was 16.7%. Myelodysplastic syndrome was concomitantly diagnosed in 10 patients (5 with p.M41T, 4 p.M41V, 1 p.M41L), and 2 additional patients had inconclusive bone marrow biopsies (p.M41V and Splice). Clinical manifestations (n=23) included constitutional symptoms (n=21, 91.3%), rashes (n=16, 69.6%), chondritis (n=6, 26.1% in 3 p.M41T, 2 splice, 1 p.M41V), inflammatory eye disease (n=6, 26.1%, including 2 patients with dacryoadenitis), thromboembolism (n=7, 30.4%, including one dying of cerebral sinus vein thrombosis, see abstract CR.517, and another with a mesenteric event). Special manifestations of interest included 7 patients with pitting edema of hands or lower limbs and 2 patients who had parotitis, symptoms which have not been previousy described VEXAS; and a patient who had pathologic evidence for renal and gastrointestinal amyloidosis. All patients were treated with prednisone (8 (34.7%) received it as a sole treatment). The maximal daily dose was 52.0±22.5 mg (15-80), and the current dose was 19.4±21.3 mg (0-80). Additional therapies included: tocilizumab (TOC), in 7 patients, (1 deceased); canakinumab in another deceased patient; azacitidine in 3 patients; ruxolitinib in 1 patient, who experienced severe cutaneous injection site reaction to subcutaneous TOC and an anaphylactic reaction to a TOC infusion. Eight patients were treated with anakinra prior to VEXAS diagnosis, and at least 5 of them had severe cutaneous injection site reactions. One patient was treated with anakinra after diagnosis, but had a flare of his Sweet-like rash and was switched to TOC. This patient had a severe cutaneous injection site reaction to a BNT162b2 vaccine. Other treatments used before and after diagnosis included sarilumab, infliximab, canakinumab, colchicine, hydroxychloroquine, methotrexate, azathioprine, leflunomide, mycophenolate, dapsone and cyclosporine. Overall, response to current therapies was partial – both in lab tests and clinically (Table 1).
Conclusion: The genetic milieu of the Israeli population may differ from other western countries. In this cross-sectional national study, we found that the UBA1 p.M41V pathogenic variant, which carries a worse prognosis, was more prevalent in Israeli Jewish patients than is reported from other cohorts around the world. This may explain the low rate of chondritis in our cohort.
REFERENCES: [1] Beck DB, et al. . NEJM . 2020;383:2628–38.
[2] Georgin-Lavialle S, et al. . BJD . doi: 10.1111/bjd.20805
[3] Ferrada MA, et al. Blood . 2022;140:1496–506.
Acknowledgements: We would like to thank Michal Rosenzwaig, MA, the Genetic Institute, Tel Aviv Sourasky Medical Center and Haike Reznik Wolf, PhD, The Danek Gertner Institute of human genetics, Sheba Medical Center for their contribution.
Disclosure of Interests: Tali Eviatar GSK, Abbvie, AstraZeneca, Abbvie, AstraZeneca, Michael Zisapel: None declared, Neta Feinstein Goren: None declared, Iftach Sagy: None declared, Shaye Kivity: None declared, Merav Lidar: None declared, Amir Bieber: None declared, Oshrat Tayer-Shifman: None declared, Hagit Peleg: None declared, David Ozeri: None declared, Jana Shamash: None declared, Elon Pras: None declared, Hagit Baris-Feldman: None declared, Yonatan Edel: None declared, Ariela Dortort Lazar: None declared, Ofir Wolach: None declared, Yair Molad: None declared, Galia Stremer: None declared, Mohammad E. Naffa: None declared, Tzika Porges: None declared, Ori Elkayam: None declared.