

Background: The ameliorative effect of pregnancy on rheumatoid arthritis (RA) was first reported in the 1930s but the cause remains unclear. CD4+CD25+FOXP3+ regulatory T cells (Tregs), which are defective in RA, have a crucial role in maintaining maternofoetal tolerance in pregnancy and their circulating number and function was previously found to correlate with amelioration of RA in the third trimester [1].
Objectives: We conducted a prospective cohort study of pregnant women with RA (RA-P) to test the hypothesis that amelioration of RA in pregnancy is associated with enhanced Treg function, which is lost in postpartum (PP) flare.
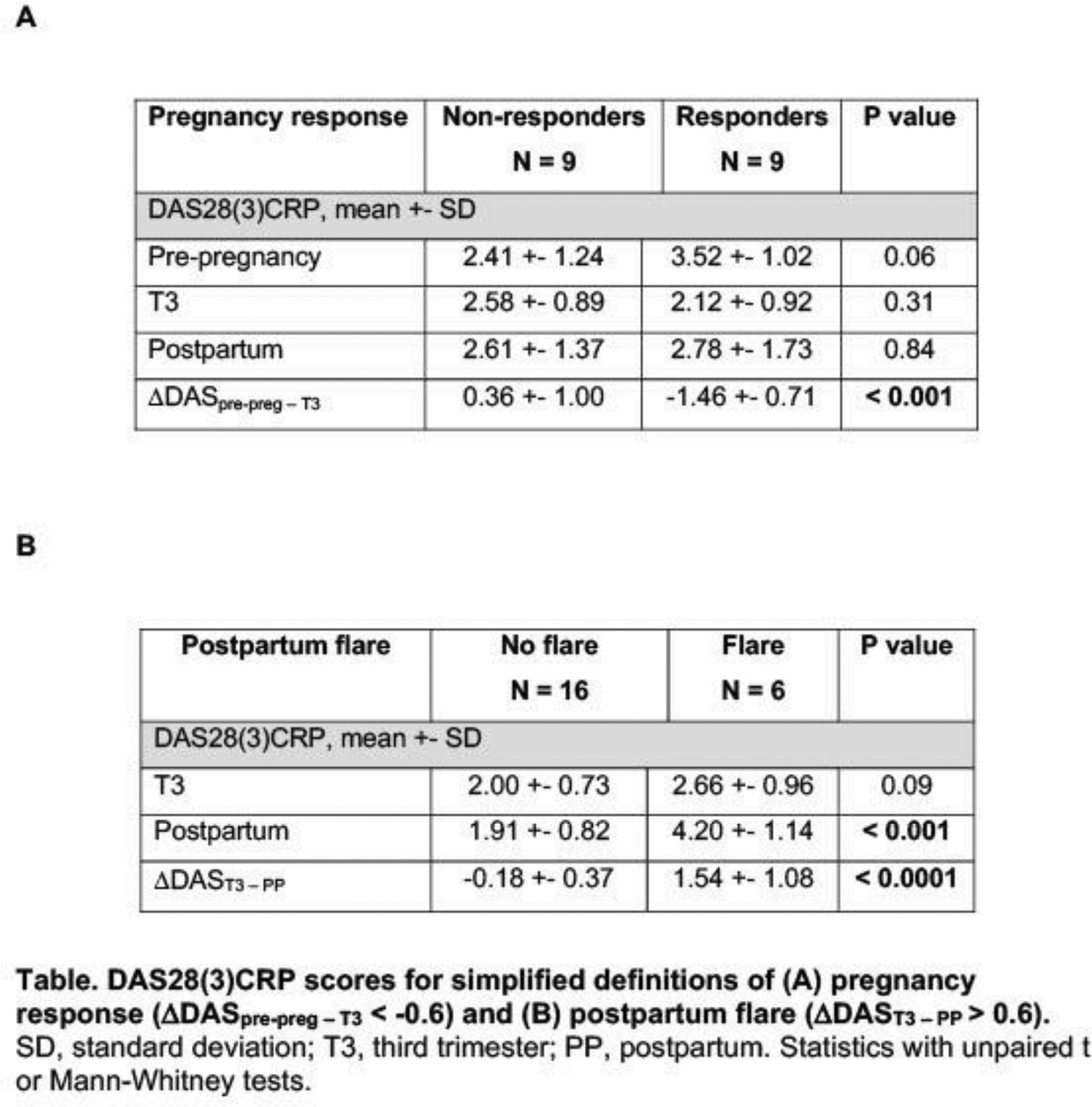
Methods: RA-P who fulfilled 2010 ACR/EULAR classification criteria were assessed at >28 weeks (T3) and within 6 months PP. RA disease activity was measured with Disease Activity Score (DAS)-28(3)CRP. Simplified definitions of disease activity response in pregnancy and PP were used based on [2]: DAS response in pregnancy was defined as: ΔDAS pre-preg–T3 <-0.6 (ΔDAS pre-preg–T3 calculated as: DAS28(3)CRP in T3 – DAS28(3)CRP pre-pregnancy (within 6 months, obtained from patient records)). PP flare was defined as ΔDAS T3 – PP >0.6 (ΔDAS T3 – PP calculated as: DAS28(3)CRP in PP – DAS28(3)CRP in T3).
Peripheral blood was collected from subjects with isolation of peripheral blood mononuclear cells (PBMCs). Multiparameter flow cytometry was used for detailed immunophenotyping of Tregs and CD4+CD25- responder T cells (Tresps). Tregs were gated on CD4+CD25+CD127-FOXP3+. Functional markers included CD69, programmed cell death-protein 1 (PD-1) and Helios (marker of thymic Tregs). Intracellular cytokine staining (transforming growth factor (TGF)-β, interferon (IFN)-γ) was undertaken after overnight stimulation of PBMCs with anti-CD3, anti-CD28 and IL-2 followed by 4 hour stimulation with PMA, ionomycin and brefeldin A. Relative Treg:Tresp activity was assessed by the ratio of expression of these markers.
Results: N=28 RA-P were recruited, mean age 34.2 years (27–42). Medication use comprised n=5 patients on no treatment; hydroxychloroquine, n=14; sulfasalazine, n=13; anti-tumour necrosis factor, n=6; anti-IL-6, anti-CD20 and CTLA-4 fusion protein n=1 each. N=4 patients on prednisolone >10 mg were excluded. Of remaining patients with available data, n=9 patients experienced DAS response in pregnancy (RA-P-R) and n=9 did not respond (RA-P-NR); n=6 patients experienced PP flare (RA-PP-F) and n=16 did not flare (RA-PP-NF), Table 1.
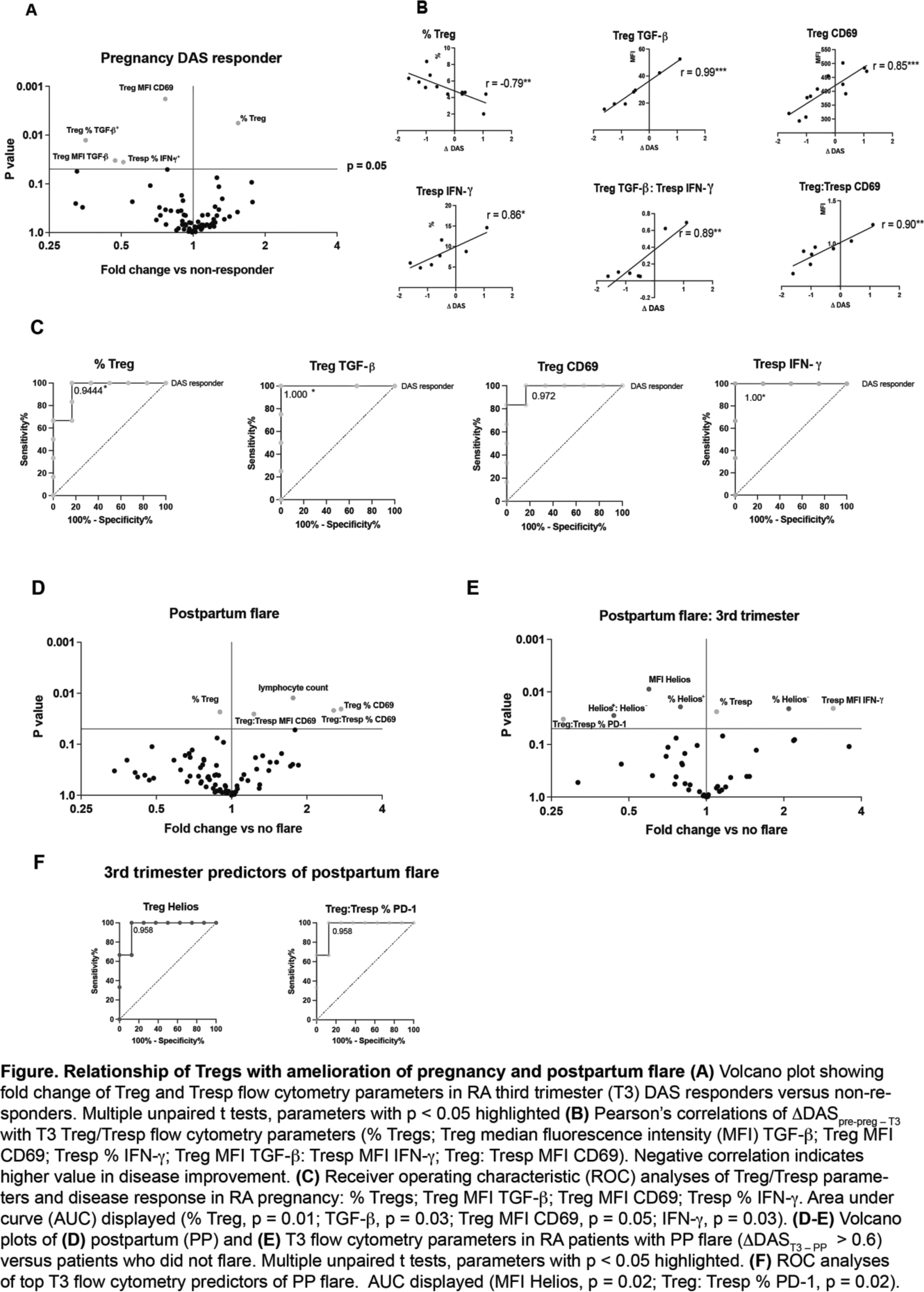
Multiple unpaired t tests found an increase in %Tregs in T3 of RA-P-R compared to RA-P-NR. However, markers of Treg function were lower in RA-P-R compared to RA-P-NR: median fluorescence intensity (MFI) CD69, % and MFI of TGF-β+ as well as Tresp % IFN-γ (p < 0.05, Figure 1A). These functional markers as well as Treg TGF-β:Tresp IFN-γ production and Treg:Tresp CD69 expression were positively correlated with ΔDAS pre-preg–T3 (Figure 1B) . Receiver operating characteristic (ROC) analyses showed that these markers were excellent predictors of DAS response in RA-P (Figure 1C).
PP flare was associated with reduced Treg but elevated total lymphocyte counts. Treg CD69 expression and Treg:Tresp CD69 expression were increased in PP flare (Figure 1D).
T3 parameters were compared in RA-PP-F versus RA-PP-NF. Tresp IFN-γ production was significantly increased at T3 in RA-PP-F (Figure 1E). The proportion and MFI of Helios + Tregs and Treg:Tresp % PD-1 were significantly reduced in RA-PP-F and predicted PP flare at T3 (Figure 1F).
In contrast to previous findings and our hypothesis, amelioration of RA in pregnancy was associated with increased number but reduced function of Tregs, and with reduced function (IFN-γ production) by Tresps.
Tregs were activated in PP flare as assessed by CD69 expression
Treg/Tresp alterations in RA-PP-F were evident at T3, with increased Tresp production of IFN-γ, Treg induction (increased proportion of Helios - Tregs), and reduced Treg: Tresp PD-1 expression. These parameters could be studied as potential predictors of PP flare in future work.
REFERENCES: [1] Forger F et al . Ann Rheum Dis. 2008;67(7):984-90.
[2] de Man YA et al . Arthritis Rheum. 2008;59(9):1241-8.
Acknowledgements: NIL.
Disclosure of Interests: Charles Raine UCB, George Robinson: None declared, Coziana Ciurtin: None declared, Jessica Manson: None declared, David Williams: None declared, Elizabeth Jury: None declared, Ian Giles: None declared