

Background: Platelets (PLT) exert an immunomodulating activity in multiple normal biological processes, such as damage repair and defence against infections. It has been clearly demonstrated that they play a significant role in promoting and supporting inflammatory and autoimmune diseases, such as rheumatoid arthritis (RA). However, the knowledge on the mechanisms through which PLT are involved in these processes are very limited.
Objectives: The purpose of this study was to in vitro investigate the effect of PLT on T helper (Th)17 axis biology in RA patients in comparisonto healthy subjects and their contribution to the pathogenesis of the disease.
Methods: CD3+ T cells were isolated and PLT ultrapurified from peripheral blood of a cohort of patients with RA diagnosed according to 2010 ACR/EULAR classification criteria, with at least moderate disease activity (DAS28-CRP>3.2) and no ongoing b/tsDMARD treatment and of a group of age and sex-matched healthy controls (HC). T cells and PLT were co-cultured at a ratio of 1:100. Both homologous (i.e., T cells and PLT from the same subject) cultures and cross-cultures (i.e., T cell from RA patient with PLT from HC and vice versa) were set up, in order to investigate whether any observed effect was caused by T cells or PLT. Cultures were carried out in the presence of anti-CD3 and anti-CD28 antibodies. At day 5, cells were harvested and the percentage of Th17 cells (CD4+IL-17A+ of CD3+) in the presence or absence of PLT was assessed by flow cytometry. T cell proliferation was evaluated at day 5 upon carboxyfluorescein succinimidyl ester (CFSE) staining, using flow cytometry and assessment of proliferation index (PI). The concentration of IL-17A in culture supernatants was also estimated at day 5 by ELISA. RNA sequencing and transcriptome analysis was performed after 18h of culture on paired T cell and T cell+PLT samples.
Results: Twelve patients with RA and twelve HC were enrolled.
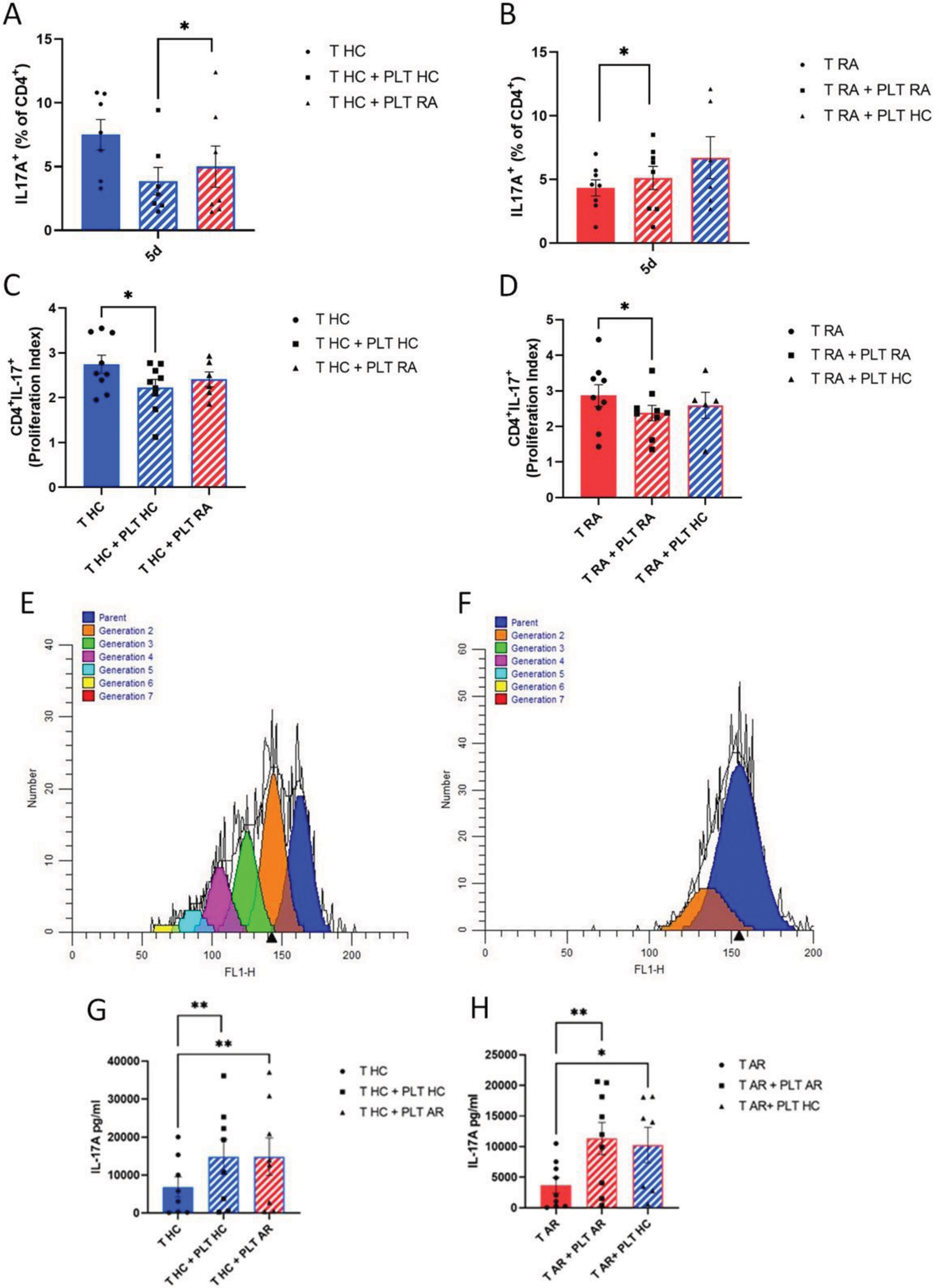
In the presence of PLT, a reduction of % Th17 cells was observed in HC at day 5 (7.5 ± 1.2% vs 3.9 ± 1.1%, p=0.016) (Figure 1A). In RA samples, no effect was observed in %Th17 cells (4.3 ± 0.6% vs 5.1 ± 0.9%, p=0.313) (Figure 1B), while there was a reduction of T cell proliferation in both RA and HC (Figure 1C–F). A significant increased concentration of IL-17A in the supernatants of cultures of both HC (6.9 ± 7.6 ng/ml vs 14.8 ± 13.9 ng/ml, p=0.008) and RA samples (3.7 ± 3.8 ng/ml vs 11.3 ± 7.9 ng/ml, p=0.004) was observed in the presence of PLT (Figure 1G and H).
Overall, the results of homologous and cross-cultures did not show significant differences, thus RNA sequencing was performed on homologous cultures only.
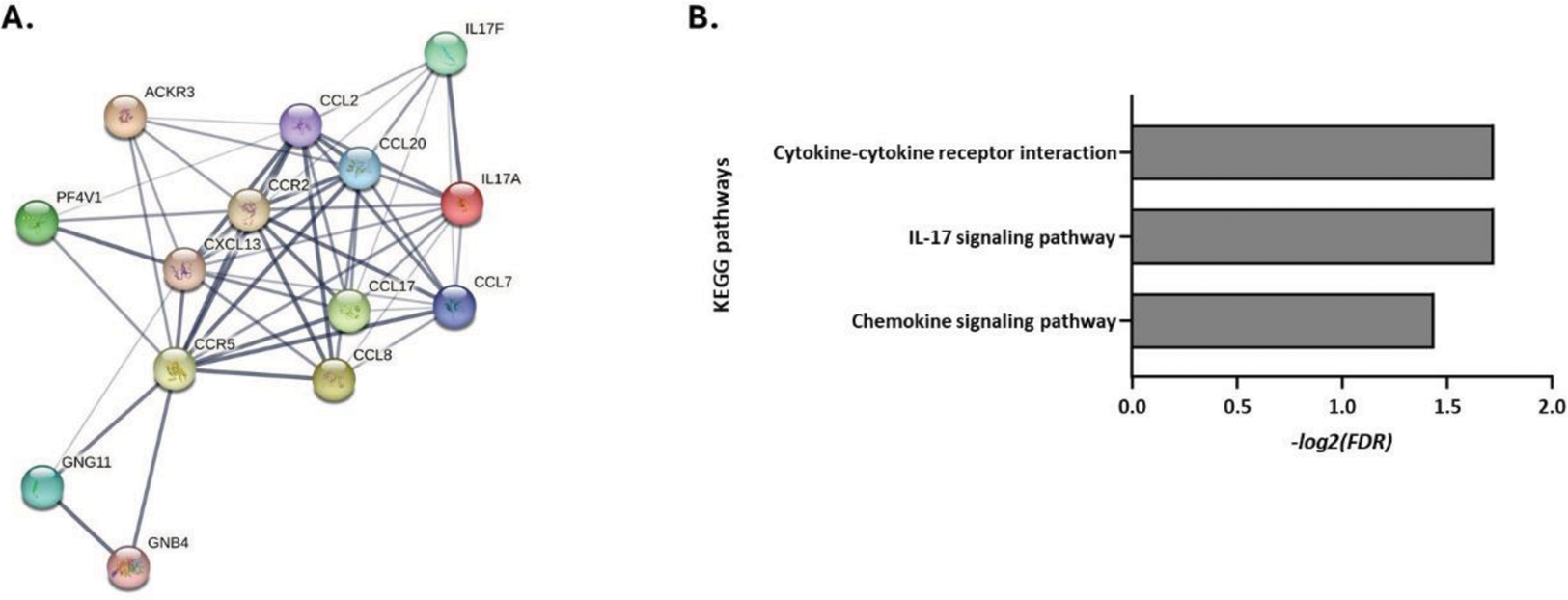
Following transcriptome analysis, 149 and 50 differentially expressed genes specific for RA and HC PLT:T cell co-culture samples were identified, respectively. Gene-set and pathway enrichment analysis revealed significant deregulation of the Th17 pathway (FDR=0.019) associated with a significant upregulation of IL-17A , IL-17F , CCL20 (p<0.05, |fold-change|>2) in RA T vs. PLT:T cell samples (Figure 2), while no corresponding significant changes were observed in HC samples.
Conclusion: The results of this study provide indications that PLT may exert a pro-inflammatory effect in RA by upregulating the transcription of Th17-pathway related genes resulting in maintenance of a Th17 cellular response that is, instead, downregulated in HC. These data, together with the absence of an increased proliferation of Th17 cells, suggest that PTL effects are mostly exerted on a differentiation level. The apparently contrasting results of IL-17 secretion can be explained by the well-known potent and unspecific initial stimulating effect of PLT on the secretion of stored intracellular IL-17, together with other cytokines, upon contact with T cells.
REFERENCES: [1] Zhu L. et al. J. Thromb. Haemost. 2014
[2] Tan, S. et al. Platelets. 2022
RA-specifically enriched KEGG pathways in PLT:T cell cultures. (A) Protein-protein-interaction network of differentially expressed genes involved in the pathways (B) Bar diagram depicting the -log2(false discovery rate, FDR) of significance of enrichment of each pathway.
Acknowledgements: NIL.
Disclosure of Interests: Giacomo Cafaro Janssen, Eli-Lilly, Maria-Ioanna Christodoulou: None declared, Sabrina Cipriani: None declared, Manuela Sebastiano: None declared, Carlo Perricone: None declared, Onelia Bistoni: None declared, Roberto Gerli: None declared, Elena Bartoloni: None declared