

Background: Ocular dryness is a hallmark feature of patients with primary Sjogren’s Disease (pSD). There is still great unmet medical need as treatment options are mainly symptomatic and directed at relieving ocular dryness and often not sufficient enough, causing a substantial burden on these patients (1, 2). In our recent trial (RepurpSS-I) with Leflunomide (LEF) Hydroxychloroquine (HCQ) combination therapy we observed a significant decrease of disease activity but no significant changes in ocular dryness (3). These results were consistent with previously published studies, demonstrating the persistence of ocular dryness upon systemic treatments. Surprisingly, we observed increased tear production in patients who experienced transient facial nerve palsy as a consequence of local anesthetic (Lidocaine) use during parotid biopsy procedures.
Objectives: To investigate if lidocaine significantly affects tear production in patients with pSD as compared to normal variation in tear production.
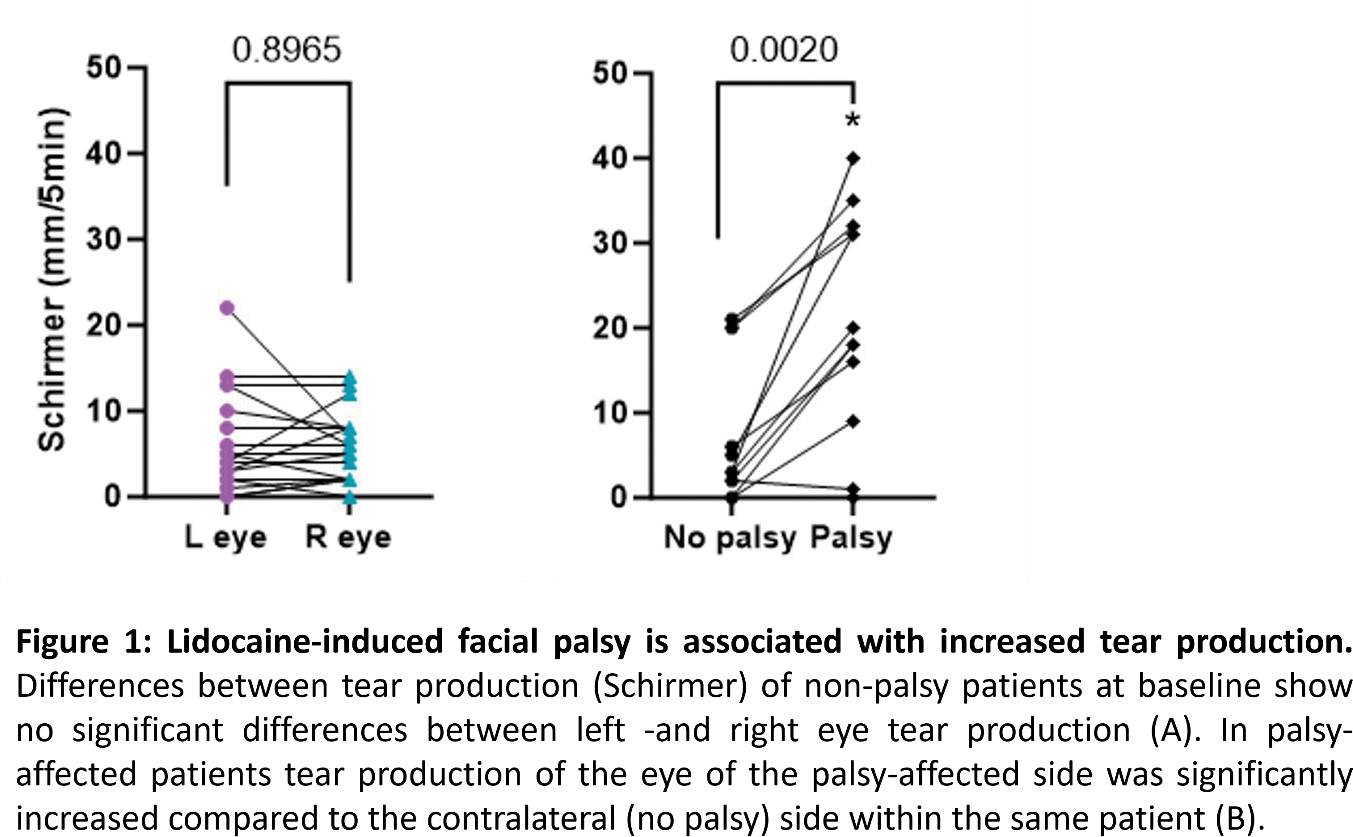
Methods: Parotid biopsies were performed at two timepoints (baseline and 24 weeks = clinical endpoint) on 29 patients included in the RepurpSS-I trial (3). Tear production was measured by Schirmer. Lidocaine-induced facial palsy occurred upon parotid biopsy on 14 occasions, in two of 12 patients palsy occurred at baseline and at week 24. Effects were related to one of two surgeons, applying higher lidocaine dosages. The outcome of the palsy-affected side was compared to the contra-lateral side within each patient. This was compared to left -and right eye differences studied at baseline in patients without facial palsy. In addition, the correlation of left -and right eye measurements was investigated to indicate normal coherence between both sides. For analysis univariate -and multivariate statistics were used.
Results: At baseline no significant within-patient differences in ocular dryness were seen between left and right eyes in the group that did not experience facial palsy (n=23, mean ± SEM left eye 5.0 ± 1.21 vs right 4.61 ± 0.910 mm, p=0.897, Figure 1A). Moreover, a significant correlation was demonstrated at baseline between left -and right eye Schirmer scores (r= 0.8365, p=<0.0001). As reported previously 3 , comparing baseline and 24-week endpoints did not reveal any significant differences between placebo and LEF/HCQ groups (mean 5.3 ± 7.4 to 3.7 ± 5.1, p=0.773 in the placebo and 5.6 ± 6.6 to 6.8 ± 11.3 mm, p=0.625 in the LEF/HCQ group, assessed using only Schirmer scores of “non-palsy” measurements). However, a significant increase in tear production was observed within-patients when the lidocaine/palsy-affected eyes were compared to their contralateral sides, irrespective of timepoint, with a mean Schirmer of 18.3 ± SEM 4.05 mm on the palsy-affected side vs 6.42 ± SEM 2.48 mm on the non-palsy side (p=0.002, Figure 1B). Furthermore, upon multivariate analysis an increased tear production in the affected (facial palsy side) eye was shown compared to non-palsy affected eyes in this group with a fixed effect of ±12.8 mm in favor of the palsy group (p<0.0001), independent of study drugs.
Conclusion: To our knowledge this is the first documented report indicating that tear production in pSD patients is increased by blocking (more central) neuronal pathways, at least temporarily relieving ocular dryness. This observed phenomenon may help in broadening the horizon for possible future treatment strategies for ocular dryness in pSD.
REFERENCES: [1] Foulks GN, Forstot SL, Donshik PC, et al . Clinical guidelines for management of dry eye associated with Sjogren disease. Ocul Surf. 2015;13(2):118-32.
[2] Michaelov E, McKenna C, Ibrahim P, et al . Sjogren’s Syndrome Associated Dry Eye: Impact on Daily Living and Adherence to Therapy. J Clin Med. 2022;11(10).
[3] van der Heijden EHM, Blokland SLM, Hillen MR, et al . Leflunomide–hydroxychloroquine combination therapy in patients with primary Sjögren’s syndrome (RepurpSS-I): a placebo-controlled, double-blinded, randomised clinical trial. The Lancet Rheumatology. 2020;2(5):e260-e9.
Acknowledgements: NIL.
Disclosure of Interests: None declared.