

Background: Interaction between T and B cells are critical for antibody production in germinal centres, but their role and immunophenotypic characterization is scarcely studied in primary antiphospholipid syndrome (pAPS). Whether antiphospholipid antibody (aPL) profile, including non-criteria aPL, dictates clinical, laboratory and immunological differences is unclear.
Objectives: To address whether pAPS patients differ clinically and immunologically based on current aPL status.
Methods: We prospectively recruited 92 adults with thrombotic PAPS (fulfilling Sidney criteria) and 75 age- and sex-matched healthy donors (HD). Clinical and laboratory data were collected at study entry. Regardless of lupus anticoagulant (LA) status, patients were categorized in two groups according to current aPL profile (IgM and/or IgG anti-cardiolipin [ACL], -ß2-glycoprotein [ß2GPI], -phosphatidylserine/prothrombin complex [PS/PT]; and IgG anti-Domain-1 of ß2GPI [D1- ß2GPI]): aPL+ , positive for at least one aPL (n=43); and aPL -, negative for all abovementioned aPL (i.e., positive for LA in the past and/or currently) (n=49). We considered aPL <30 UQ as negative (ELISA immunoassays). Circulating Tfh (CD4 + FoxP 3 - CD25 - CD45RO + CXCR5 + ), activated Tfh (PD1 + ICOS + Tfh), Treg (CD4 + CD25 + FoxP 3 + ), Tfr (CD4 + CD25 + FoxP 3 + CXCR5 + ), naïve B cells (CD19 + CD27 - IgD + ), pre- (CD19 + CD27 + IgD + ) and post-switch memory B cells (CD19 + CD27 + IgD - ), transitional B cells (CD19 + IgD + CD38 ++ ) and plasmablasts (CD19 + CD27 + IgD - CD38 ++ ) were analysed by flow cytometry.
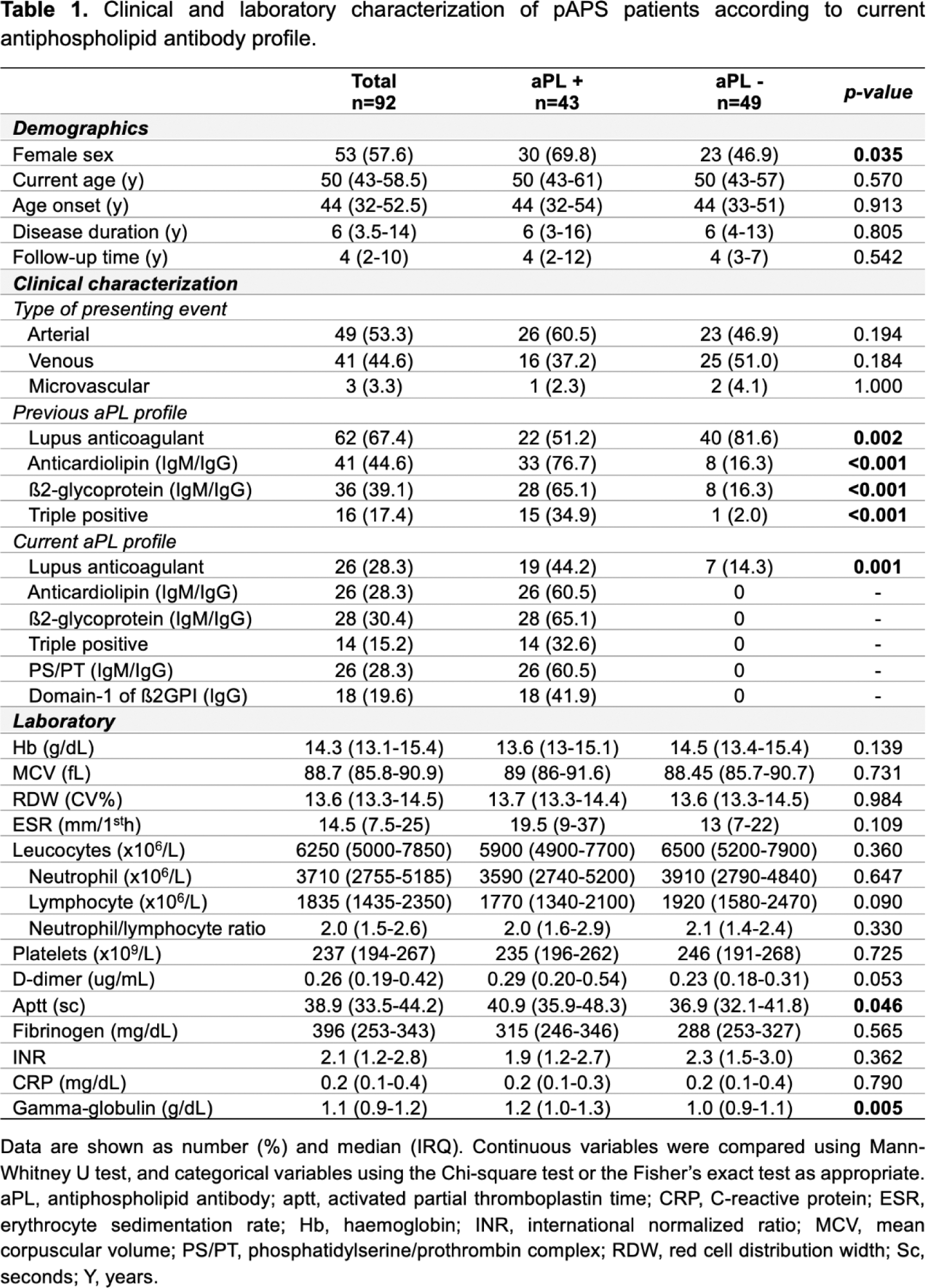
Results: Most patients were women (n=53, 57.6%), with a median (IQR) age at disease onset of 44 (32-52.5) years and a median disease duration of 6 (3.5-14) years ( Table 1 ). Arterial thrombosis was the most common first thrombotic manifestation (51.3%). Prevalence of criteria-aPL positivity decreased from diagnosis to follow-up, respectively ( Table 1 ): LA (59.8%; 28.3%); ACL (47.8%; 28.3%); ß2GPI (40.2%; 30.4%). Non-criteria-aPL anti-PS/PT (IgM/IgG) and anti-D1-ß2GPI (IgG) were currently present in 28.3% and 19.6% of patients, respectively. Current age, disease duration, type of first thrombotic event, INR, d-dimers, erythrocyte sedimentation rate and c-reactive protein were comparable between aPL+ and aPL - pAPS patients ( Table 1 ). Only activated partial thromboplastin (p=0.046) time and gamma-globulin values (p=0.005) were increased in aPL+ patients ( Table 1 ). The frequencies of circulating Tfh and Treg cells were comparable between HD and pAPS patients ( Figure 1A and 1B ). Within the Tfh subset, activated Tfh cells were significantly increase in pAPS compared to HD (p=0.046), and in aPL+ compared to aPL - patients (p=0.037) ( Figure 1C ). Within the Treg subset, circulating Tfr and Tfr/Tfh ratio were significantly increased in aPL+ patients compared to aPL- patients (p<0.001; p=0.034) and HD (p<0.001) ( Figure 1D and 1E ). The frequencies of circulating naïve B cells, pre- and post-switch B cells, transitional B cells and plamablasts were similarly distributed between pAPS patients, HD, and specific aPL profile subgroups ( Figure 1F to 1J ).
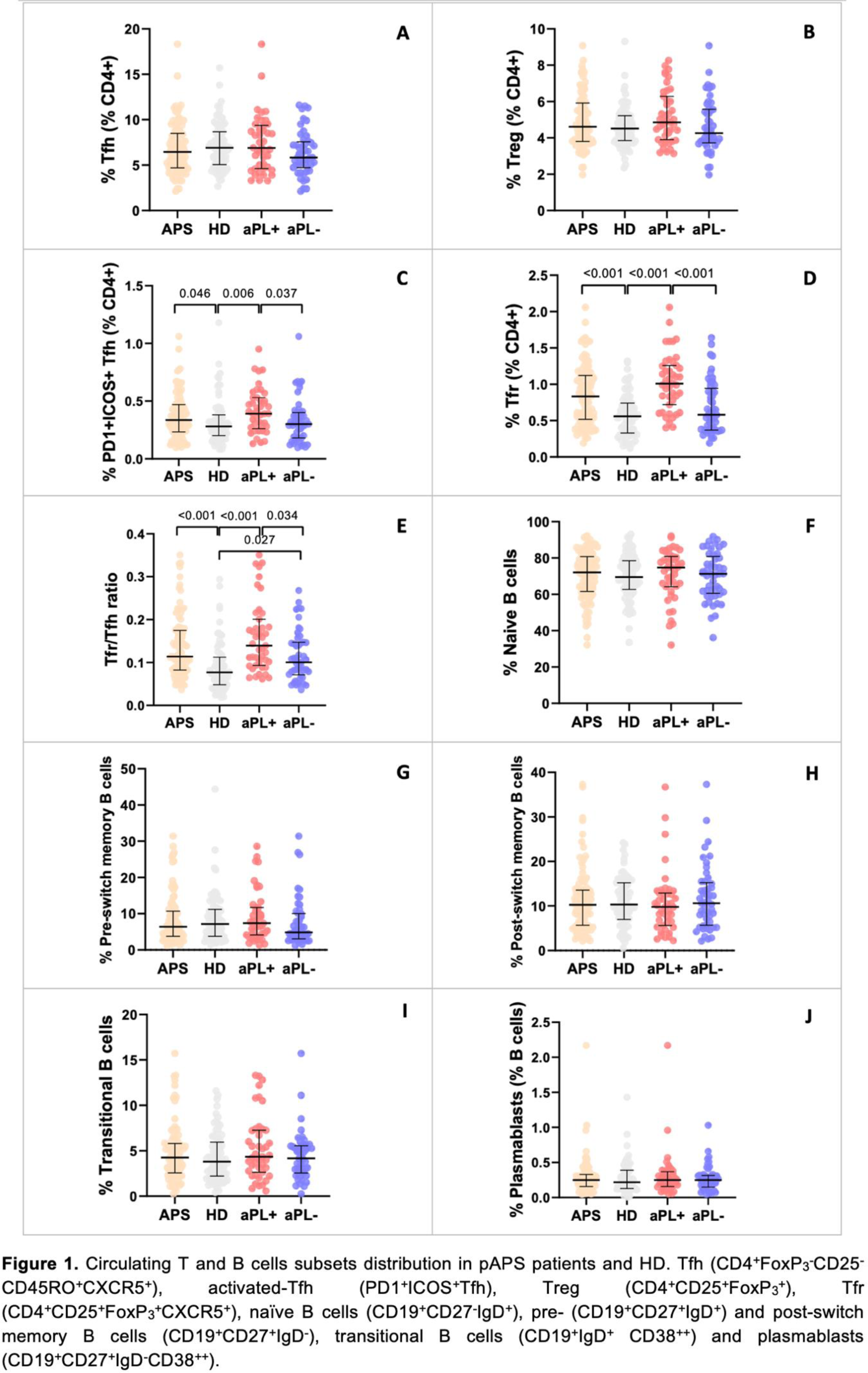
Conclusion: Despite clinical and laboratory similarities among thrombotic aPL+ and aPL - pAPS patients, an imbalance of circulating Tfh and Tfr subsets, but not B cells, was noted according to aPL profile. Increased activated Tfh and Tfr cells, and Tfr/Tfh ratio might indicate an active state of disease characterized by ongoing humoral response in aPL+ patients. aPL - patients displayed a similar immunological T and B cell profile compared to healthy individuals.
REFERENCES: NIL.
Acknowledgements: This work is funded by National Funds through the Portuguese Funding agency FCT - Fundação para a Ciência e Tecnologia, I.P.
Disclosure of Interests: None declared.