

Background: To determine if a CD19.CAR-T-cell combination is safe and effective in severe SSc-ILD.
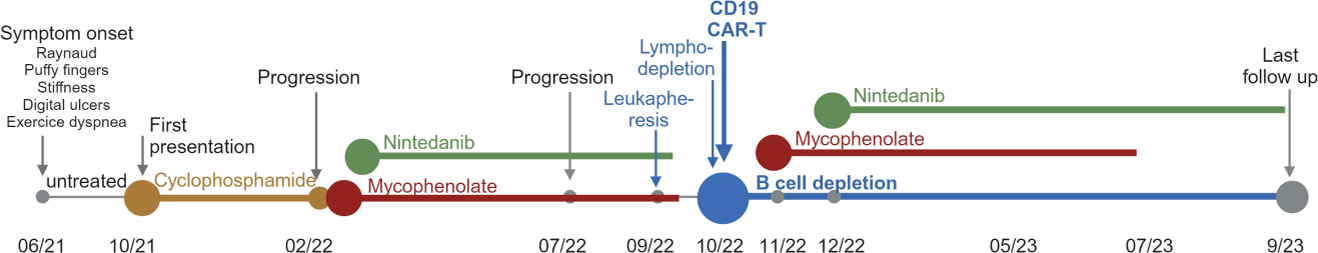
Case presentation: The longitudinal case study was conducted at the University Hospital Heidelberg. An Scl70+ SSc-ILD case with progressive pulmonary fibrosis (NSIP) and fatal prognosis was followed for 2 years. The patient received 3 rd -generation CD19.CAR-T-cells [1] as compassionate use for the first time in a non-cancer patient and in combination with mycophenolate and nintedanib (Figure 1). The longitudinal study included exploratory analyses of the bioactivity of circulating immune complexes at Fcγ-receptors using a new reporter cell model [ 2-4] and of phenotypes of immune cells by spectral flow cytometry. We observed a delayed and sustained clinical amelioration after the addition of CAR-T-cells to mycophenolate/nintedanib. At around month 6 and enduring until the last follow up (month 11), dyspnea, pulmonary function and CT findings have improved dramatically. Chronically elevated CRP levels normalized. mRSS decreased from 22 to 11. Regarding side effects, a temporary pulmonary deterioration after lymphodepletion therapy and CAR-T-infusion occurred. No severe adverse event were observed. During the disease course, mycophenolate and nintedanib incompletely reduced circulating immune complexes that activate Fcγ-receptors. After addition of CAR-T cells, systemic activity of immune complexes was no longer detectable. CAR-T cells were also associated with an altered immune landscape including a rejuvenation of Fcγ-receptor-bearing NK cells.
Learning points for clinical practice: This case study suggests safety and unprecedented efficacy of this CAR-T combination in severe SSc-ILD. Hypothetically, disappearing circulating immune complexes and recovering FcγR-bearing cells could contribute to the great success of CAR-T cells in systemic autoimmune diseases.
REFERENCES: [1] Schubert et al., J Hematol Oncol, 2023.
[2] Zhao et al., Arthritis Rheumatol, 2022.
[3] Chen et al., EMBO Mol Med, 2022.
[4] Ankerhold al., Nat Commun, 2022.
Acknowledgements: We thank Anja Funkert, Stefan Krienke and further staff of the Medizinische Klinik V and Thoraxklinik, Heidelberg, including the team of the GMP Core Facility and all physicians, involved in the manufacturing of the 3rd generation CD19.CAR-T-cells, in the treatment of the patient and the sampling of materials or data. Funding: Deutsche Gesellschaft für Innere Medizin e.V. (to WM).
Disclosure of Interests: Wolfgang Merkt WM has received consulting fees, speaking fees, support for meetings and/or travel and/or honoraria from Novartis, Roche, UCB, BMS and Galapagos and unrestricted third-party funds from Roche between 2015 and 2018., Merle Freitag: None declared, Maren Claus: None declared, Philipp Kolb: None declared, Manuel Röhrich: None declared, Lea Rodon: None declared, Ivana Andreeva: None declared, Franca Deicher: None declared, Norbert Blank NB has received honoraria and meeting support from SOBI, Novartis and Boehringer., Carsten Watzl: None declared, Markus Polke payment or honoraria for lectures, presentations, speakers’ bureaus from AstraZeneca and Boehringer Ingelheim, Claus Heussel: None declared, Hanns-Martin Lorenz has received grants from Abbvie, Novartis, Pfizer, Roche/Chugai and consulting fees, honoraria, meeting support from Abbvie, Astra-Zeneca, Actelion, Amgen, Bayer Vital, Boehringer Ingelheim, BMS, Celgene, GSK, Gilead/Galapagos, Janssen-Cilag, Lilly, Medac, MSD, Novartis, Pfizer, Roche/Chugai, Sanofi, UCB., Michael Schmitt research grants from Apogenix, Hexal and Novartis. Travel grants from Hexal and Kite. Financial support for educational activities and conferences from bluebird bio, Kite and Novartis. Advisory board member of MSD. (Co-)PI of clinical trials of MSD, GSK, Kite and BMS. Co-Founder and shareholder of TolerogenixX Ltd.