

Background: Recently, the interest and research in artificial intelligence (AI) in healthcare has increased exponentially [1, 2]. AI encompass the development of computer systems or software that can perform tasks that typically require human intelligence [3]. These tasks include learning, reasoning, problem-solving, understanding natural language, speech recognition, and visual perception [3]. But, what is the landscape of AI in Rheumatology? What type of tasks is AI comprising in two of the areas with greater research, namely rheumatoid arthritis (RA) and spondyloarthritis (SpA)? What is the level of validation and adequacy?
Objectives: To analyse the uses, type of research and its quality, of AI in RA and SpA.
Methods: We performed a systematic review (SR) using a standardised, reproducible approach, following the Cochrane dual-reviewer methodology, and observing the PRISMA statement (PROSPERO CRD42023396462). The protocol was established by a group of rheumatologists experts in digital health tools, who defined the research question (PICO) and the data to be collected. We searched for articles that analysed the characteristics, efficacy, safety or validity of any digital health technology in RA and SpA (including psoriatic arthritis; PsA). Two expert Librarians established a sensitive search strategy in Medline, Embase and the Cochrane databases (up to December 2022), using MeSH and free text terms. By study type, SRs, randomized controlled trials (RCTs) and observational studies were eligible. A hand search was completed by reviewing the references of the included studies, plus publications and other information provided by the experts. Two reviewers selected the studies and collected the data. To grade the quality of included studies, we used the PROBAST (Prediction model Risk Of Bias Assessment Tool) and AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) tools. The information gathered included country, data source, application of AI, and type of algorithms.
Results: The global search included 560 studies of which 84 met the criteria. Two were SRs, 8 RCTs and 74 observational studies. Most of the included studies were carried out in the United States of America. We found a great variability by type of AI, data sources, and studied samples. By type of AI algorithm, 34 studies used multiple models, 10 artificial neural networks, and 10 random forest. By disease, 54 studies were focused on RA, 14 on axSpA/pSpA, of which 6 were on PsA and 10 on rheumatic diseases in general but including patients with RA or SpA. The applications of AI were medical diagnosis in 21 studies, prognosis in 39 and prediction of treatment response in 24. No implementation studies were found.
Regarding the quality of the AI-based models, we identified several limitations that hinder reliable assessment of the models including the lack of specific guidelines about an objective methodology for the design and validation of the model, lack of flexibility, the handling of many variables, and the underreporting of results (see Figure 1 for detailed quality according to the PROBAST scale).
PROBAST quality scale
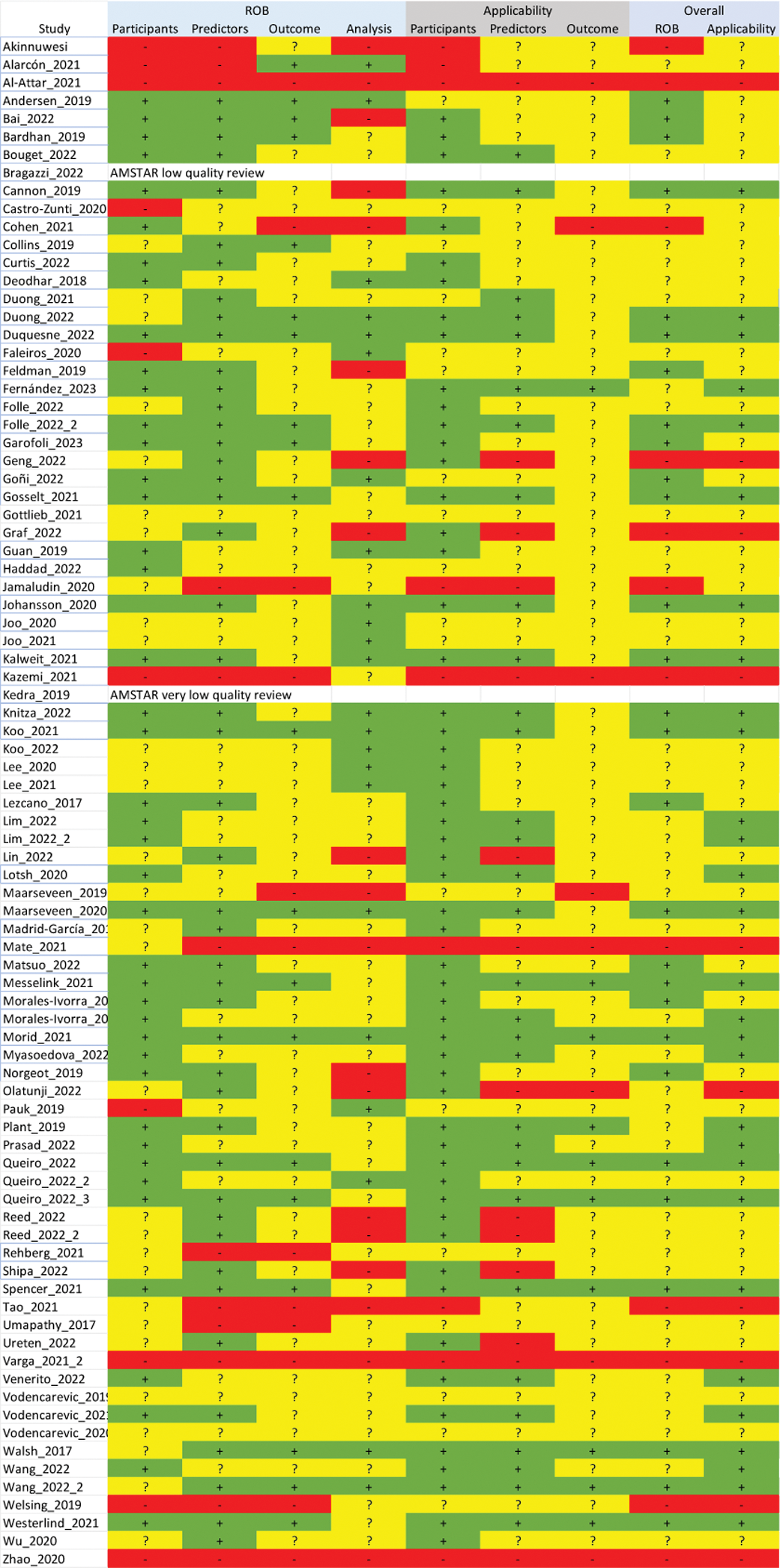
Conclusion: Numerous AI-based models and applications have been developed for RA and SpA, especially in the areas of diagnosis, prognosis and treatment response; however, they still show deficiencies, highlighting the need for better critical appraisal.
REFERENCES: [1] Bergier H, Duron L, Sordet C, Kawka L, Schlencker A, Chasset F, et al. Digital health, big data and smart technologies for the care of patients with systemic autoimmune diseases: Where do we stand? Autoimmun Rev. 2021;20(8):102864.
[2] Solomon DH, Rudin RS. Digital health technologies: opportunities and challenges in rheumatology. Nat Rev Rheumatol. 2020;16(9):525-35.
[3] Russell S, Norvig P. Artificial Intelligence: A Modern Approach: Prentice Hall Press; 2009.
Acknowledgements: NIL.
Disclosure of Interests: Diego Benavent: None declared, Loreto Carmona: None declared, Jose Francisco Garcia LLorente: None declared, Maria Montoro Full-time employee of Pfizer, SUSAN RAMIREZ Full-time employee of Pfizer, Teresa Oton: None declared, Estíbaliz Loza: None declared, Antonio Gómez-Centeno: None declared