

Background: Neuropsychiatric lupus affects the central and peripheral nervous system. Physiopathology includes a blood-brain barrier disruption that leads to the infiltration of autoantibodies and immune cells into the brain [1]. Vitamin D (VitD) has immunoregulatory properties that have been explored in autoimmune diseases [1]. Pristane-induced lupus (PIL) presents multiple classification criteria of SLE, but the neuropsychiatric manifestations are poorly studied [2]. The role of VitD in this context is unknown.
Objectives: To characterize cerebral immunopathological changes and IFN-α1 serum levels in the pristane-induced lupus model, as well as to assess the impact of VitD supplementation on such observations.
Methods: Seventy-six BALB/c mice were randomized into 3 groups: CO (Control, n=24), PIL (induced at T0 with 500μL of pristane intraperitoneally, n=28) and VD (PIL with VitD supplementation [2ug/kg] every two days subcutaneously, n=28). Brain tissue was collected and frozen following euthanasia 90 (n=38) and 180 (n=38) days after induction. Brain IgG and IgM deposits and pVDR expression were evaluated through immunofluorescence and IFN-α1 serum levels through ELISA. Statistical analyzes were performed in the SPSS program. Kruskal-Wallis test and ANOVA were used. Data are presented as mean±standard deviation. P value was considered ≤0.05.
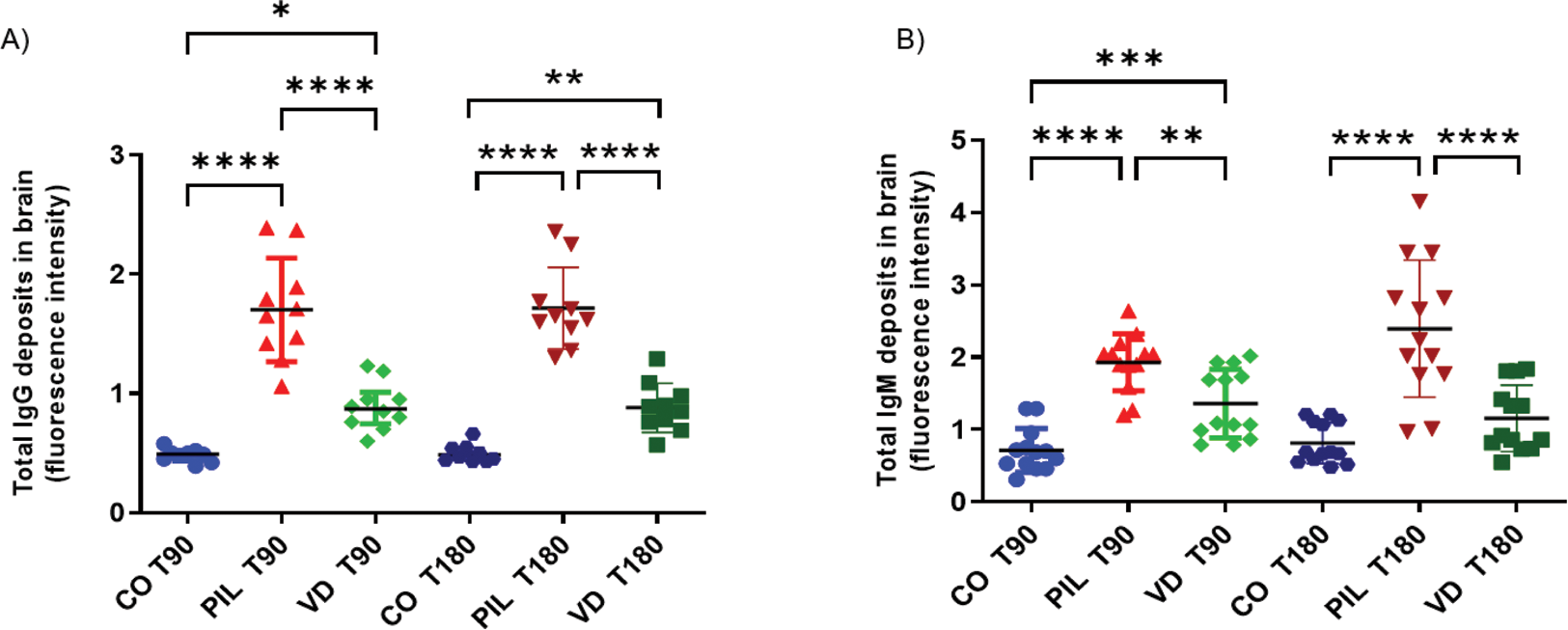
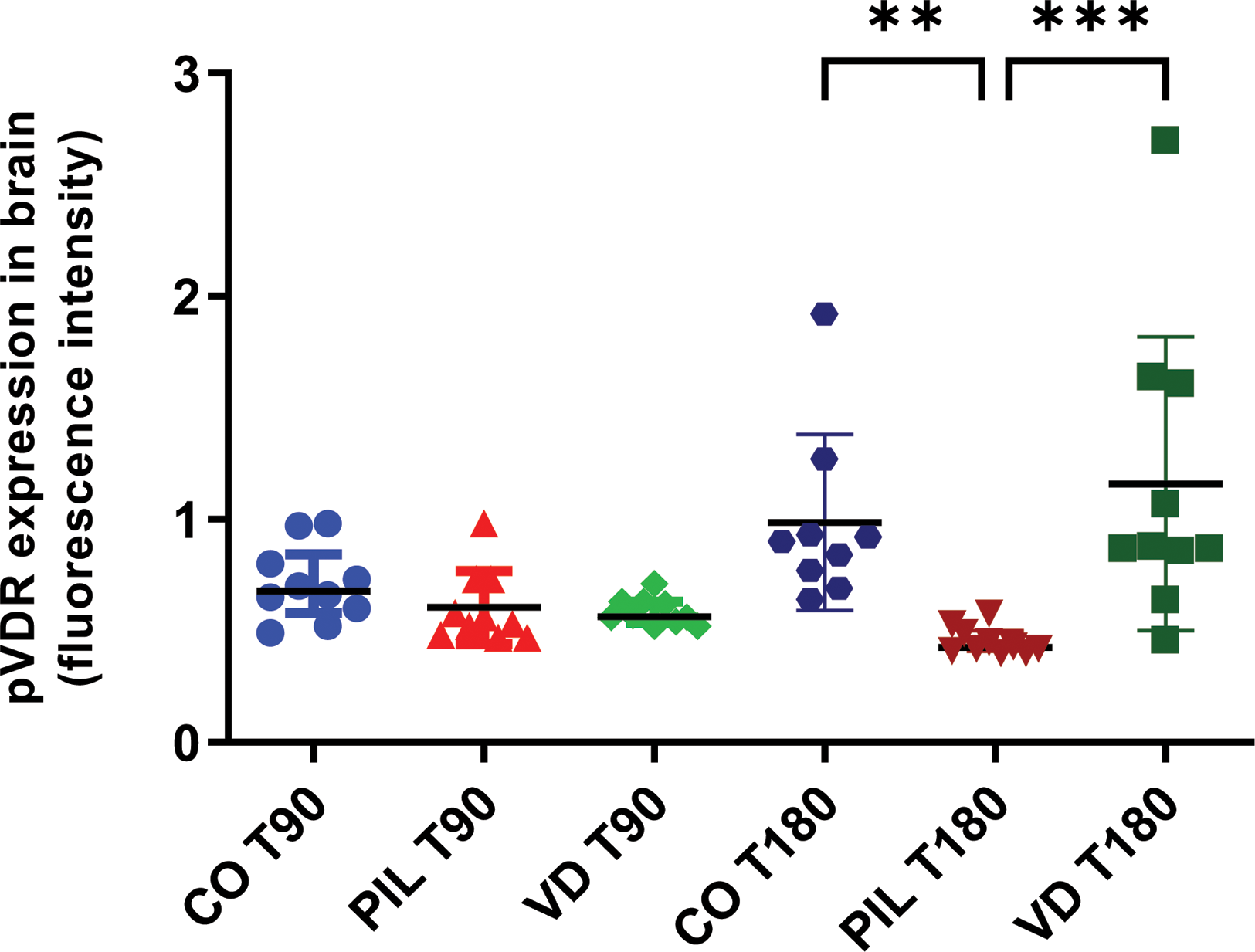
Results: PIL group exhibited a significant increase in total IgG and IgM deposits in the brain compared with the CO group at T90 (IgG: 1.70±0.43 vs. 0.48±0.05, p<0.0001; IgM: 1.92±0.39 vs. 0.71±0.30, p<0.0001) and T180 (IgG: 1.71±0.34 vs. 0.49±0.07, p<0.0001; IgM: 2.39±0.94 vs. 0.81±0.28, p<0.0001). Supplementation with VitD reduced both IgG (T90: 0.89±0.19, p<0.0001; T180: 0.88±0.20, p<0.0001) and IgM (T90: 1.35±0.47, p<0.01; T180: 1.15±0.45, p<0.0001) deposits at both time points (Figure 1). At T90, pVDR expression was similar across groups (CO: 0.71±0.16; PIL: 0.60±0.16; VD: 0.58±0.06). However, at T180, an increase in pVDR expression was observed in the CO and VD groups (CO: 0.98±0.39; PIL: 0.45±0.06; VD: 1.16±0.65, p<0.01 PIL vs. CO; p<0.001 PIL vs. VD) (Figure 2). Serum levels of IFN-α1 were elevated in both PIL and VD groups compared to the CO group (T90: CO: 5.50±2.60; PIL: 13.74±3.36; VD: 13.07±5.55, p=0.03; T180: CO: 3.22±0.41; PIL: 11.63±3.85; VD: 11.45±3.77, p=0.001). VitD supplementation did not reduce IFN-α1 serum levels.
Total IgG and IgM deposits in the brain in a pristane-induced lupus model. A) Total IgG fluorescence intensity in brain regions. B) Total IgG fluorescence intensity in brain regions. Values are expressed as mean±SD. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. CO3: controls 3 months after induction; PIL3: pristane-induced lupus 3 months after induction; VD3: pristane-induced lupus+vitamin D 3 months after induction; CO6: controls 6 months after induction; PIL6: pristane-induced lupus 6 months after induction; VD6: pristane-induced lupus+vitamin D 6 months after induction.
pVDR expression fluorescence intensity in brain regions. Values are expressed as mean±SD. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. CO3: controls 3 months after induction; PIL3: pristane-induced lupus 3 months after induction; VD3: pristane-induced lupus+vitamin D 3 months after induction; CO6: controls 6 months after induction; PIL6: pristane-induced lupus 6 months after induction; VD6: pristane-induced lupus+vitamin D 6 months after induction.
Conclusion: PIL model presented increased deposits of IgG and IgM in the brain tissue. VitD was able to reduce this immunopathological finding and enhance pVDR expression in the VD group after 180 days of treatment. PIL exhibited elevated levels of IFN-α1, which VitD did not modulate. Further data analysis is required to comprehend the immunological effects and clinical implications of VitD in this murine PIL model.
REFERENCES: [1] Karnopp TE, Freitas EC, Rieger A, Chapacais GF, Monticielo OA. Clin Rheumatol 2022. doi: 10.1007/s10067-022-06094-2
[2] Freitas EC, de Oliveira MS, Monticielo OA. Clin Rheumatol 2017. doi: 10.1007/s10067-017-3811-6
Acknowledgements: Douglas dos Santos Soares (Laboratório de Pesquisa Cardiovascular at HCPA); Unidade de Experimentação Animal (HCPA); Diretoria de Pesquisa (HCPA); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Disclosure of Interests: None declared.