

Background: B cell depletion is widely used to treat SLE. Rituximab (RTX) is already widely used, and other agents such as obinutuzumab, and CD19 CAR-T are being developed. However, B cells have diverse roles in SLE, including immunostimulatory and immunoregulatory roles. The mechanism of action of these therapies is not fully understood, nor do we know how to identify patients suitable for these therapies. Clinical and imaging data from a recent trial in SLE arthritis (ROOTS) suggested a two-phase response pattern with initial worsening compared to a placebo, followed by later benefits.
Objectives: To analyse serum samples from ROOTS to understand the effects of B cell depletion on the serum proteome at early (4 weeks) and late (16 weeks) time points.
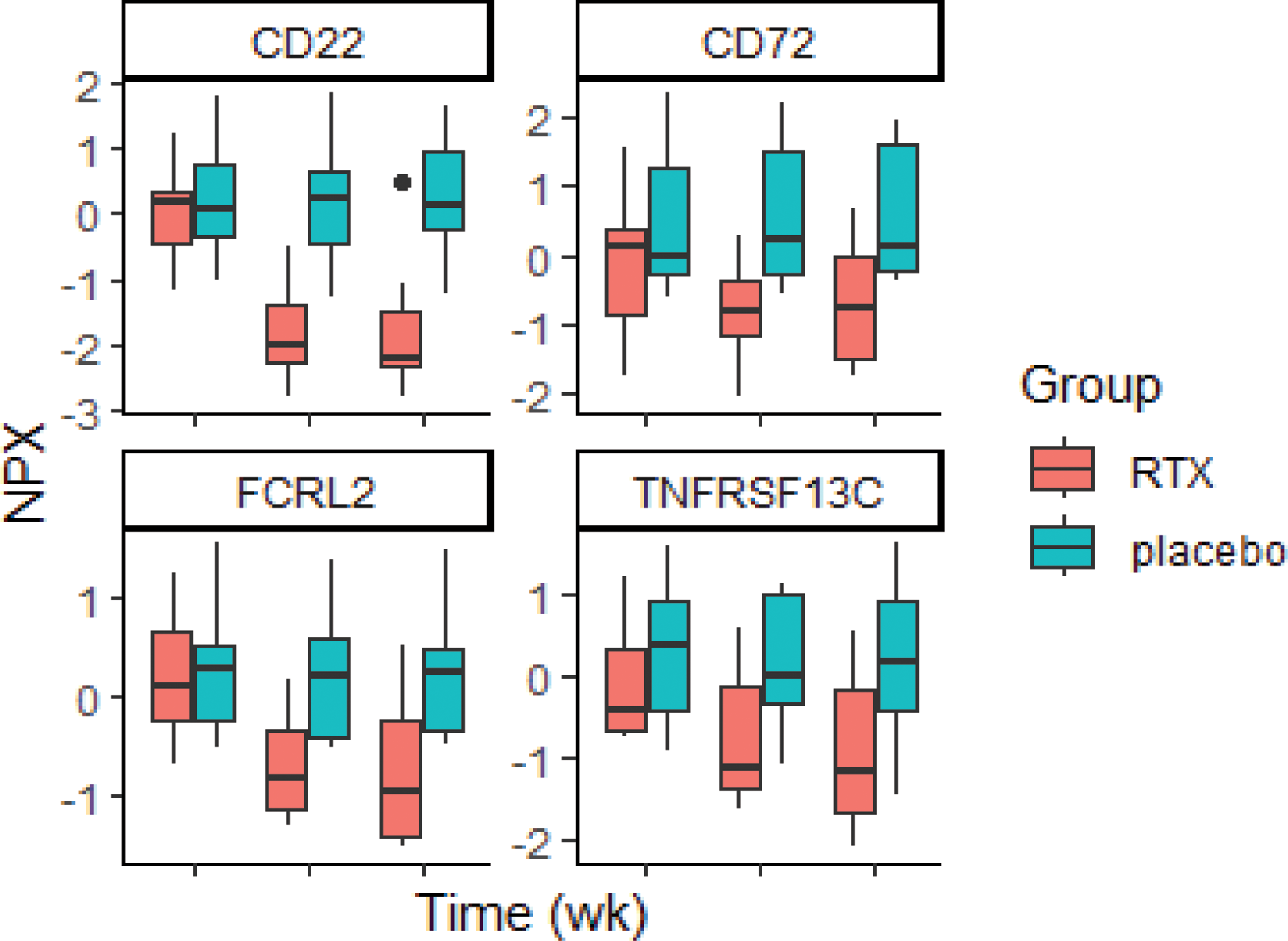
Methods: Adults with SLE were enrolled if they had clinical synovitis and/or ultrasound (US) tenosynovitis and/or positive power Doppler (PD) in ≥1 joint despite stable background therapies, including a maximum of 10 mg prednisolone. Baseline characteristics are shown in Table 1. Patients were randomized to 1000mg RTX or placebo, on days 1 and 15. Blinded infusions were preceded by 100 mg methylprednisolone. Serum was stored at 0, 4 and 16 weeks, and the relevant proteins were analysed from 764 proteins in EXPLORE Inflammation I and II panels using Proximity Extension Assay (PEA) technology (Olink®, Uppsala, Sweden). Samples were compared between treatment groups at week 0, week 4, and week 16 were analysed. Protein quantification was reported in-log10 Normalized Protein eXpression (NPX) (adjust p-value) relative protein quantification unit on a log2 scale and analysed using the OlinkAnalyze package in R.
Results: 27 participants were randomised, of whom 25 completed the study, 14 in the RTX group and 11 in the placebo group. There were no differences between the baseline characteristics of the groups (Table 1). The proteins significantly different after adjustment for multiplicity were the B cell receptor-related proteins, including CD22, CD72, FCRL2, and BAFF-R, which had significant changes at weeks 4 and 16 in RTX-treated patients compared to placebo (Figure 1). In addition, several other immune proteins displayed substantive differences between rituximab and placebo; B cell-related proteins, including IL4 and APOL1, were substantively higher at both weeks 4 and 16. At week 4, rituximab-treated patients had higher levels of the IFN-I-related protein SIGLEC1, while lower levels of other IFN-I-related proteins, CXCL9 and 10, at week 16. At week 16 but not week 4, RTX-treated patients also had substantively lower levels of other proteins involved in the immunopathology of SLE, including IL31, IFN gamma and IL12.
Conclusion: This study showed consistent and biologically plausible patterns of B cell and IFN-I-related protein quantification that appeared to support our hypothesis of a two-phase immune response to B cell depletion. Our results suggest an increase in IFN-I pathway activation at week 4 and then a decrease at week 16. Other non-B cells and non-IFN-I proteins related to SLE immunopathology were only normalised at later time points in RTX-treated patients. Collectively, our proteomic, clinical and imaging data suggest that RTX does indeed cause a transient worsening of SLE disease activity prior to improvement and that B cell-depleting monoclonal antibodies or cellular therapies, therefore, should be used in combination with glucocorticoids and other therapies, particularly in patients with severe disease activity.
REFERENCES: NIL.
Table 1. Baseline characteristic of SLE patients in rituximab and placebo groups
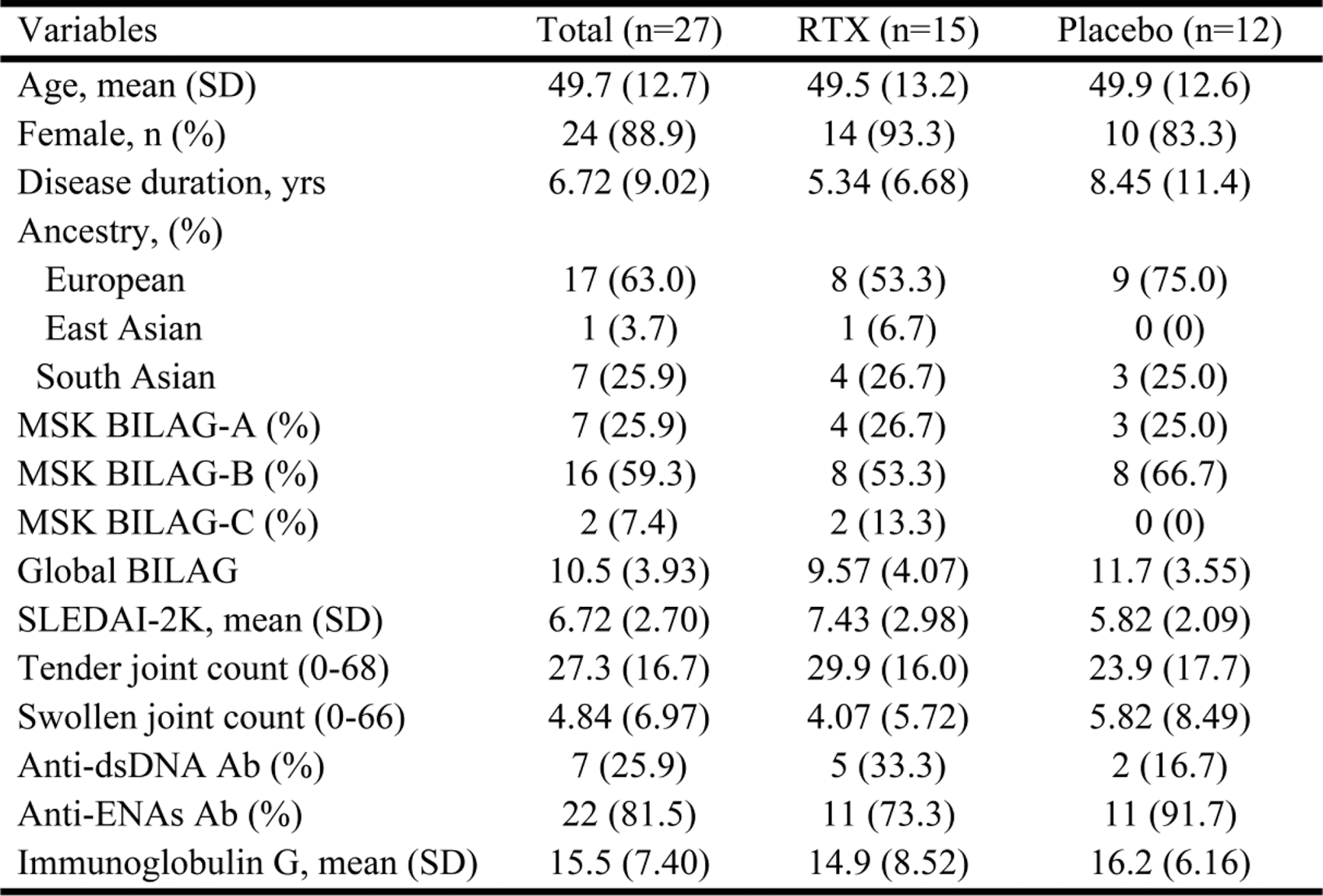
Boxplot demonstrates the significant B-cells and IFN-I-related quantified proteins between the rituximab and placebo at different times.
Acknowledgements: NIL.
Disclosure of Interests: Porntip Intapiboon Novatis,Amgen, Lucy M. Carter Alumis, Jack Arnold Alumis, Khaled Mahmoud: None declared, Md Yuzaiful Md Yusof Roche, Novartis and Alumis., Aurinia Pharmaceuticals and UCB, Edward M. Vital Roche, GSK, AstraZeneca, Aurinia Pharmaceuticals, Lilly and Novartis., Roche, AstraZeneca and Sandoz.