

Background: Systemic lupus erythematosus (SLE) is complex disease. Mice homozygous for the lymphoproliferation spontaneous mutation ( Fas lpr ) showing systemic autoimmunity are used for studying SLE. The lpr mutation is in the Fas gene, resulting in ineffective Fas-mediated apoptosis that leads to nuclear autoantibody production and ultimately end-organ damage including kidney. NLRP12 (NLR family pyrin domain containing 12) primarily expressed by myeloid cell lineage, was recently identified as an essential negative regulator of innate immune pathways. The expression level of NLRP12 is downregulated by nuclei acids, type I IFN and SLE serum. Neutrophil extracellular traps (NETs) is a unique form of inflammatory cell lysis, also named NETosis, in which neutrophils can die by expelling chromatin forming a web-like structures of DNA embedded with the histone proteins as well as the antimicrobial proteins such as neutrophil elastase (NE) and myeloperoxidase (MPO). The NETs itself is initiated through the influx of calcium, leading to activation of kinase and activation of the NADPH oxidase signaling cascade.
Objectives: To validate the role of Nlrp12 with NETs formation.
Methods: We generated autoimmune-prone mice by intercrossing B6.MRL- Fas lpr/lpr /J mice with Nlrp12 -/ - mice (C57BL6J). The offspring of those mice were Nlrp12 -/ - and Fas lpr homozygous (hereafter referred to as Nlrp12 -/ - /lpr) and Nlrp12 +/+ Fas lpr/lpr (referred to as WT/lpr). Mice were sacrificed in 7 and 9 months to compare the clinical index and neutrophils were seperated for detecting the capacity of NETs formation.
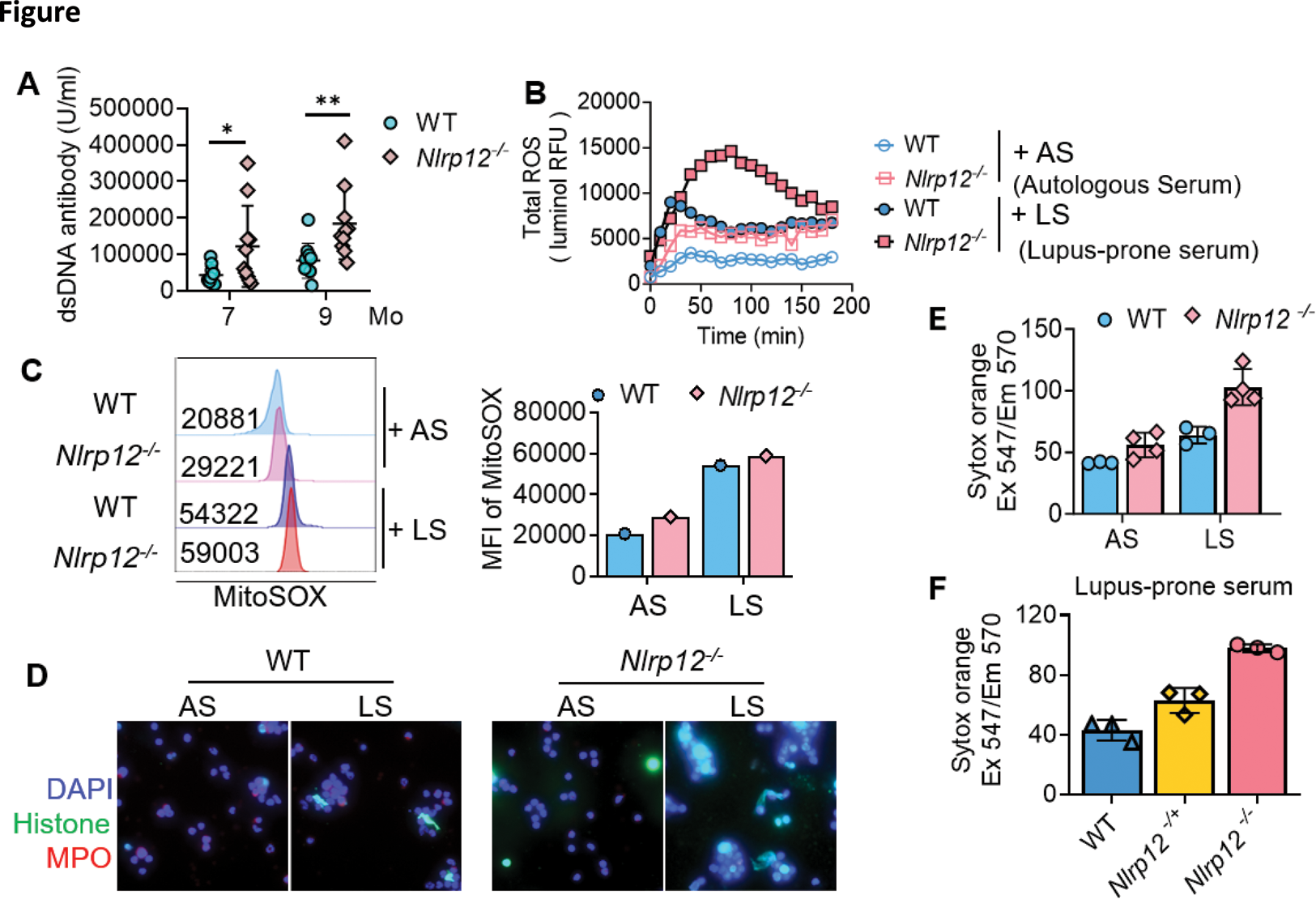
Results: Anti-dsDNA antibody titer was examined from 7 and 9 months old mice of WT/lpr and Nlrp12 -/ - /lpr, and Nlrp12 -/ - /lpr mice revealed significantly higher level of anti-dsDNA in comparison with WT/lpr (Figure 1A). We collected neutrophils from 8 weeks of WT/lpr and Nlrp12 -/ - /lpr mice and stimulated with 10% Nlrp12 -/ - /lpr pooling serum from 7 to 9 months as an IC-containing stimulator to detect total ROS formation by luminal reagent. The higher ROS production was noted in neutrophils of Nlrp12 -/ - /lpr mice, reflecting the susceptibility of neutrophils when Nlrp12 deficient (Figure 1B). The similar result of more ROS production in Nlrp12 -/ - /lpr neutrophils after 10% IC-containing serum stimulation from lupus-prone mice could be observed by MitoSOX with flowcytometry (Figure 1C). NETs formation was conducted after 4 hours treatment of 10% serum from lupus-prone mice by immunofluorescence staining with histone (red) and MPO (green), which showed more NETs formation in Nlrp12 -/ - /lpr neutrophils (Figure 1D). Sytox orange was used to the DNA component releasing from NETs. After incubation with 10% serum from lupus-prone mice and 8-week-old mice autologous serum by 4 hours, more NETs could be observed from Nlrp12 -/ - /lpr neutrophils, especially after stimulation by serum from lupus-prone mice (Figure 1E). The NETs formation after simulation by high IC containing serum by Sytox orange increased gradually from WT/lpr , Nlrp1 +/ - /lpr to Nlrp12 -/ - /lpr neutrophils (Figure 1F), indicating the effect and dose dependent of Nlrp12 in contribution to the vulnerability of NETs formation.
Conclusion: In our study, the more deficient of Nlrp12 expression, the more prominent of NETs formation was noted.
REFERENCES: [1] Tsao, Y.P., et al., NLRP12 is an innate immune checkpoint for repressing IFN signatures and attenuating lupus nephritis progression. J Clin Invest, 2023. 133(3).
[2] Kaul, A., et al., Systemic lupus erythematosus. Nat Rev Dis Primers, 2016. 2: p. 16039.
[3] Németh, T., M. Sperandio, and A. Mócsai, Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov, 2020. 19(4): p. 253-275.
[4] Brinkmann, V., et al., Neutrophil extracellular traps kill bacteria. Science, 2004. 303(5663): p. 1532-5.
Acknowledgements: NIL.
Disclosure of Interests: None declared.