

Background: In systemic lupus erythematosus (SLE), targeting both type I interferon (IFN) and B-cell pathways should be a promising therapeutic approach since each provides independent and additive contributions to pathology across a diverse spectrum of patients. Upadacitinib (UPA) is a Janus kinase (JAK) inhibitor acting at several receptors both directly and indirectly transmitting IFN signals. Elsubrutinib inhibits Bruton’s tyrosine kinase (BTK) associated with B-cell signaling. A phase 2 SLE trial (NCT03978520) of UPA, elsubrutinib, or combination (ABBV-599) found that both UPA 30 mg once daily (QD) and ABBV-599 met the 24-week endpoint of SLE Responder Index 4 (SRI-4). However, in the overall population, efficacy of UPA alone was comparable to ABBV-599.
Objectives: To characterize the mechanism of action of UPA and ABBV-599 in patients with SLE.
Methods: From this randomized, double-blind, phase 2 SLE trial blood was collected at baseline, and weeks 2, 12, 24, and 48. RNAseq analysis was compared from 147 patients who received placebo, UPA (30mg QD), or ABBV-599 (elsubrutinib 60mg + UPA 30mg QD). Limma mixed-model analyses were used to determine significant differentially expressed genes between timepoints and to predict responders/non-responders to UPA, ABBV-599 high dose, or placebo. Weighted gene co-expression network analysis (WGCNA) was used to construct gene networks and to determine the modulation (significance by paired Wilcoxon test) of these networks. Total immunoglobulin G (IgG), immunoglobulin M (IgM), and anti-double-stranded DNA (anti-dsDNA) IgG concentrations were measured from serum using a commercially available immunoturbidimetric assay and enzyme linked immunosorbent assay. Immune cell subsets and counts were identified using flow cytometry.
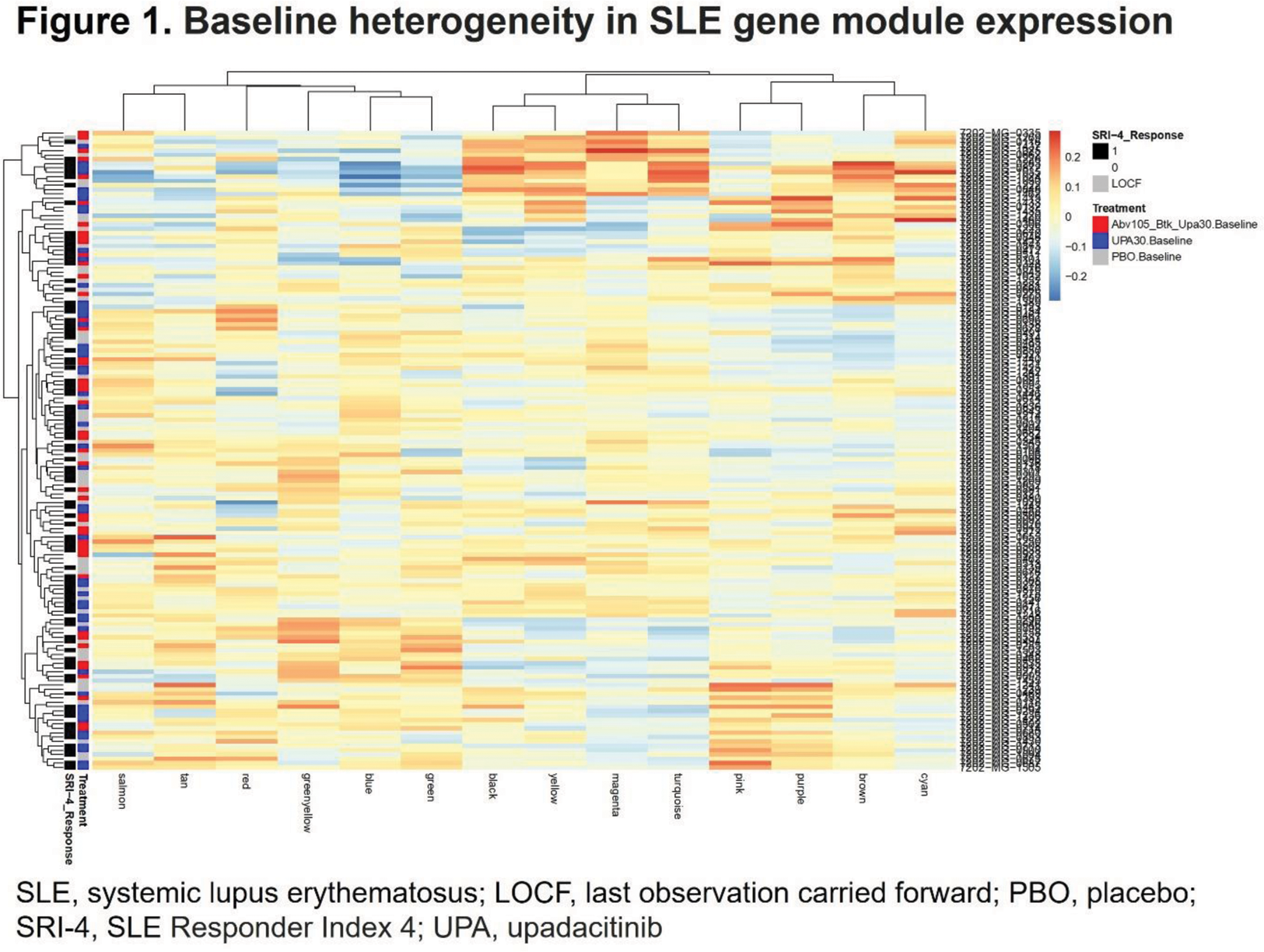
Results: Differentially expressed genes (FDR <.05) for all timepoints compared with baseline were detected for both UPA and ABBV-599 high dose, but not placebo. Distinct differences between UPA and ABBV-599 in the number of differentially expressed genes at all timepoints suggested unique mechanisms for the drugs. WGCNA of the 147 baseline samples formed 14 modules of highly correlated genes and 12 of these modules overlapped previously identified WGCNA-derived SLE gene modules perturbed in the blood of patients with SLE compared with healthy controls. Module trait correlation demonstrated significant relationships between module scores and clinical traits ( P <.01; R >.3), but not with response to treatment, in agreement with baseline heterogeneity of gene module expression for responders demonstrated by hierarchical clustering (Figure 1). The change in module eigengene values demonstrated that the BTK inhibitor led to a significant increase in neutrophil module scores, and flow cytometry demonstrated a significant increase in the percentage of neutrophils in ABBV-599, but not UPA–treated patients. However, ABBV-599 did not demonstrate a unique effect on B-cell modules, with comparable significant impacts of both UPA and ABBV-599 on post-baseline decrease in total IgG, anti-dsDNA antibodies and increase in B cells by flow cytometry. As expected, UPA significantly changed gene module values for type I IFN compared with placebo and also decreased the expression of basophil, cell cycle, and cytotoxic T-cell gene modules.
Conclusion: ABBV-599 increased neutrophil and other myeloid cell gene module scores compared to UPA, but this effect could have neutral impact related to its role in neutrophil extravasation. The BTK inhibitor in combination with UPA had little additional effect on B cells compared to UPA alone. In addition to the expected decreased expression of type I IFN gene modules, UPA treatment was associated with decreased basophil, cytotoxic T-cell, and cell cycle gene modules accounting for its efficacy in SLE patients with different baseline gene expression patterns.
REFERENCES: NIL.
Acknowledgements: AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the abstract. All authors had access to relevant data and participated in the drafting, review, and approval of this abstract. No honoraria or payments were made for authorship. AbbVie and authors thank all the trial investigators and the patients who participated in the study.
Disclosure of Interests: Joan Merrill JTM has served as a consultant and/or scientific advisor for AbbVie, Amgen, Aurinia, AZ, BMS, EMD Serono, Genentech, Gilead, GSK, Lily, Merck, Pfizer, Provention, Remegen, Sanofi, Takeda, UCB, and Zenas., JTM has received research support from AZ, BMS, and GSK., Michelle D Catalina MDC is a full-time employee of AbbVie, and may hold AbbVie stock or stock options., Ioan Filip IF is a full-time employee of AbbVie, and may hold AbbVie stock or stock options., Archana Iyer AI is a full-time employee of AbbVie, and may hold AbbVie stock or stock options., Peter K Wung PKW is a full-time employee of AbbVie, and may hold AbbVie stock or stock options., Kristin D’Silva KDS is a full-time employee of AbbVie, and may hold AbbVie stock or stock options., Marie-Claude Gaudreau M-CG is a full-time employee of AbbVie, and may hold AbbVie stock or stock options.