

Background: Rheumatoid arthritis (RA) is a rheumatic disease that is caused by autoimmune inflammatory factors, leading to increased susceptibility of joint swelling and stiffness, as well as pain, synovitis and cartilage damage [1]. Disulfidptosis is a newly identified form of cell death activated by SLC7A11-triggered disulfide stress [2]. Existing evidence shows that disulfidptosis might be involved in the pathological development of RA [3, 4]. However, the underlying functions of disulfidptosis regulators in RA remain unknown.
Objectives: This study aimed to investigate the involvement of disulfidptosis regulators in identifying molecular subtypes and uncovering potential diagnostic biomarkers of RA.
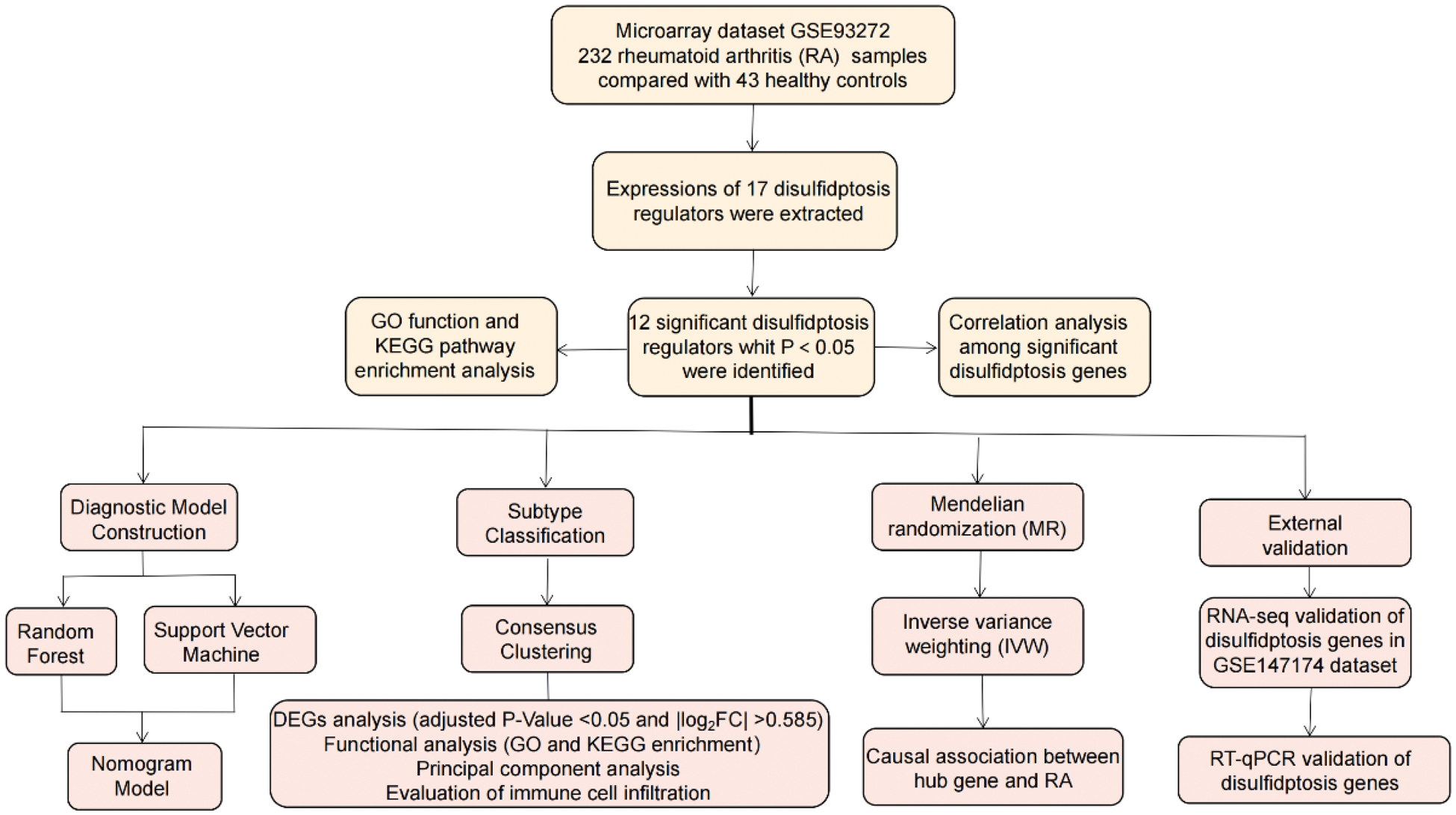
Methods: GSE93272 dataset was screened to comprehensively investigate the disulfidptosis-related diagnostic clusters and immune landscapes in RA. Moreover, bone marrow-derived macrophages (BMMs) with or without RANKL intervention were harvested for RNA sequencing (RNA-seq) to verify disulfidptosis hub gene expressions. The process flowchart for the study design was shown in Figure 1.
Results: Difference analysis between RA samples and controls screened 12 significant disulfidptosis regulators. Random forest algorithm for RA risk prediction was identify twelve diagnostic disulfidptosis regulators. Then a nomogram model of the 12 diagnostic disulfidptosis regulators was developed, and decision curve analysis suggested its potential utility in clinical practice. Additionally, consensus clustering analysis of these hub genes categorized RA patients to different disulfidptosis subgroups (clusterA and clusterB). Patients from clusterA exhibited lower disulfidptosis scores than those of patients in clusterB. Notably, we found the significant association between disulfidptosis clusters and IL6R, which have strong relationship with osteoclast differentiation. In inverse variance weighted method, we observed that IL6R was negatively associated with the risk of RA with an OR of 0.955 (95% CI = 0.926-0.984, p=3.01E-03). Remarkably, RNA-seq from GSE147174 dataset and RT-qPCR validation of disulfidptosis genes confirmed the expression trend of disulfidptosis regulators.
Conclusion: Our research on disulfidptosis patterns may provide a promising marker and an immunotherapeutical strategy for future RA therapy.
REFERENCES: [1] P. Luo, F.Q. Gao, W. Sun, J.Y. Li, C. Wang, Q.Y. Zhang, Z.Z. Li, P. Xu, Activatable fluorescent probes for imaging and diagnosis of rheumatoid arthritis, Mil Med Res, 10 (2023) 31.
[2] X. Liu, L. Nie, Y. Zhang, Y. Yan, C. Wang, M. Colic, K. Olszewski, A. Horbath, X. Chen, G. Lei, C. Mao, S. Wu, L. Zhuang, M.V. Poyurovsky, M. James You, T. Hart, D.D. Billadeau, J. Chen, B. Gan, Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis, Nat Cell Biol, (2023).
[3] H.S. Jin, J. Kim, S. Park, E. Park, B.Y. Kim, V.N. Choi, Y.H. Yoo, B.T. Kim, S.Y. Jeong, Association of the I264T variant in the sulfide quinone reductase-like (SQRDL) gene with osteoporosis in Korean postmenopausal women, PLoS One, 10 (2015) e0135285.
[4] J. Yang, R. Tang, J. Yi, Y. Chen, X. Li, T. Yu, J. Fei, Diallyl disulfide alleviates inflammatory osteolysis by suppressing osteoclastogenesis via NF-kappaB-NFATc1 signal pathway, FASEB J, 33 (2019) 7261-7273.
Flow chart of the study design.
Acknowledgements: The project was generously supported by the grants from 2024 Doctoral Student Innovation Capacity Enhancement Project of “Open Bidding for Selecting the Best Candidates”from Guangzhou University of Chinese Medicine (D-5-05).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (