

Background: Janus Kinase inhibitors (JAKi) have become an established therapeutic option for managing various immune mediated inflammatory conditions, including rheumatic and musculoskeletal diseases (RMDs). Greater treatment experience for established indications and emerging evidence highlights potential new indications and the availability of new and generic JAKi may further shape future treatment strategies. Understanding how JAKi usage may evolve will aid understanding of the future treatment paradigm, identify evidence gaps and address unmet medical needs. Research is needed to guide the direction of RMD management in the coming years.
Objectives: This work aimed to establish a global expert consensus on the role of JAKi in managing RMDs, to guide healthcare professionals (HCPs) and identify priorities for future research. The research focused on two questions: how the use of JAKi is evolving within their current approved indications and where their application might expand to include new potential indications.
Methods: A four-round modified Delphi study was conducted with panellists from 23 countries representing diverse income tiers, healthcare systems and World Health Organisation (WHO) regions. Eligibility criteria included fluency in English, clinician providing Rheumatology care, ≥ 5years Rheumatology clinical experience/or ≥5 publications) and membership of a rheumatology association. The study was guided by an Academic Working Group comprising four global experts from the UK and the US, alongside a Steering Committee with 18 representatives, one from each participating country. A scoping review [1] on published literature of JAKi use in RMDs was conducted to inform initial statements, supplemented by clinical expertise of the committee. Panellists ranked statements on a Likert scale from 1 to 9, provided feedback on statement stringency, and free text suggestions. Stable consensus or stable disagreement was defined as a median score of ≥7 or ≤3, respectively, from two consecutive rounds. All 79 statements were presented in a minimum of two rounds, with wording refined by the committee based on panellists’ suggestions.
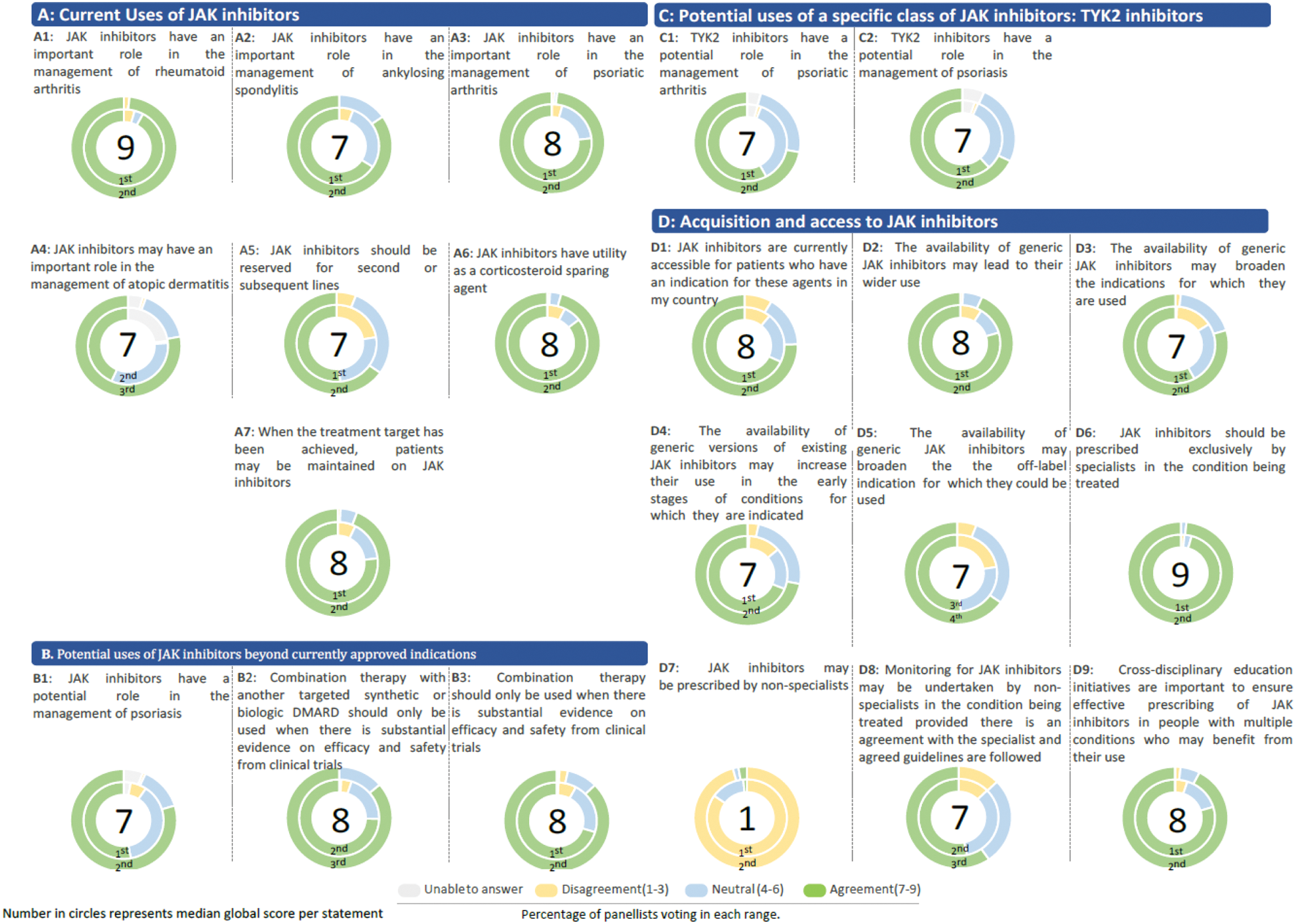
Results: A total of 178 panellists voluntarily participated in the study, representing the following WHO regions: Americas (n=30), Europe (n=40), Eastern Mediterranean (n=35), South-East Asia (n=19), Africa (n=27), and Western Pacific (n=27). Responses were 78%, 66%, 61% and 63% of total recruited panellists in rounds one to four, respectively (Figure 1). Statements were grouped into the following categories: Current Uses of JAKi, Potential uses of JAKi beyond currently approved indications, Potential uses of a specific class of JAKi: TYK2 inhibitors or Acquisition and access to JAKis (Figure 2). Of the 79 statements presented to panellists, 20 reached stable consensus, and one reached stable disagreement, with >250 free-text comments by panellists. The majority of statements which reached consensus did so in the first two rounds. Global Delphi consensus results showed strong concordance with the use of JAKi to treat rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and atopic dermatitis, although they were reserved for second and subsequent lines of treatment. Panellists strongly supported the utility of JAKi as corticosteroid-sparing agents. However, their potential significance in managing conditions for which these agents have yet to be approved was found to be considerably more limited. Global consensus was achieved for psoriasis only (of the 12 conditions identified by the scoping review [1]) with great heterogeneity in responses between countries. Clinicians were hesitant to use JAKi in the absence of phase 3 RCT evidence or equivalent. Panellists emphasised the importance of generic JAKs, agreeing that their availability could broaden their current therapeutic applications, both by allowing their use in earlier lines of treatment and by their use expanding to other indications. Notably, while the accessibility of JAKi reached overall consensus despite some regional variations, the assessment of their affordability yielded more divergent results. Opinion stratification across key differentiators including geographical region, national income and treatment practice highlighted great heterogeneity in responses, particularly in relation to cost, JAKi adoption in earlier stages of indicated conditions, and potential expansion of their use more broadly.
Conclusion: Based on available literature evidence and global expert consensus, this work highlights present and potential future trends in the use of JAKi for RMDs. While the global rheumatology community recognise the importance of JAKi in managing RMDs, they seem to need further research data before expanding the use of these agents to currently unlicensed indications. The availability of generic options may enhance accessibility and broaden treatment applications, but regional disparities in affordability remain a challenge.
This study was sponsored by Pfizer. Pfizer employees and academic authors designed the study, interpreted data, and wrote the abstract. Analytical and writing support was provided by Momentum data Ltd, funded by Pfizer.
REFERENCES: [1] Challoumas D, Simpson C, Arnold M, Mease P, Moots R, Ndosi M, Rutter Locher Z (2025). Janus-kinase inhibitor use in immune-mediated inflammatory diseases beyond licensed indications: A scoping review.
Autoimmunity
Reviews 24 (3);
Global, geographical distribution of panellists
Detailed outcomes for statements achieving stable consensus in the overall global analysis.
Acknowledgements: International steering committee and panellists who took part in the study.
Disclosure of Interests: Anna Barkaway Shareholder of Pfizer Ltd., Employee of Pfizer Ltd., Philip J. Mease Honoraria from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, UCB, Consultant for AbbVie, Acelyrin, Amgen, Bristol Myers Squib, Cullinan Biotech, Eli Lilly, lnmagene, Janssen, Moonlake, Novartis, Pfizer, Takeda, UCB, Other: Genascence, Grants/Research support from: AbbVie, Acelyrin, Amgen, Bristol Myers Squib, Eli Lilly, Janssen, Novartis, Pfizer, UCB, Robert Moots Consultant for Pfizer, Grants from Novartis, Mwidimi Ndosi Consultant for Pfizer, Grants from Sanofi and Vifor Pharmaceuticals, Zoe Rutter-Locher Consultant for Pfizer, Michael McLean Shareholder of Pfizer Ltd., Employee of Pfizer Ltd.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (