

Background: Lysophosphatidic Acid (LPA) is a lipid mediator implicated in the pathogenesis of SSc and idiopathic pulmonary fibrosis. Phase 2 clinical trials show potential benefit with attenuation of downstream antifibrotic pathways through inhibition of LPA receptor 1 (LPAR1) or autotaxin.
Objectives: We performed gene set enrichment analysis of skin biopsies from well characterised systemic sclerosis (SSc) patients and healthy controls using a validated 61 gene set published by the Broad Institute.
Methods: The BIOPSY cohort recruited 68 well characterised SSc patients prospectively from a large tertiary centre over 24 months. 50 SSc skin biopsy samples were collected across disease subsets including early diffuse cutaneous (dc)SSc (n=21), limited cutaneous (lc)SSc (n=15) and established dcSSc (n=14) with 16 matched healthy controls. Bulk RNA sequencing was used to perform analyses including genome-wide transcriptome profiling of whole blood and whole skin samples. Statistical analyses were performed using Morpheus, Microsoft Excel, GraphPad Prism and STRING software packages.
Results: Differential gene expression analysis of the published LPA gene set was used as a reference to interrogate the BIOPSY cohort subsets of SSc compared to healthy controls. Top differentially expressed LPA regulated genes by statistical significance (p value <0.05) were identified for each SSc subset. Unsupervised hierarchical clustering analysis revealed distinct gene signatures across the subsets of SSc (Figure 1). Functional analysis revealed biological pathways including D5 Dopamine receptor binding (GNA12, GNA13), calcium-independent protein kinase C activity (PRKCD and PRKCE) and LPA receptor activity (LPAR1, LPAR2, LPAR3, LPAR4).
Conclusion: Our findings confirm differential activation of LPA regulated genes across the SSc subsets that are not present in healthy skin which show significant heterogeneity in the downstream biological pathways mediated by LPA genes in SSc. Our findings support therapeutic targeting of this pathway in SSc and identify subgroups and may inform future clinical study design and help understand differential treatment response.
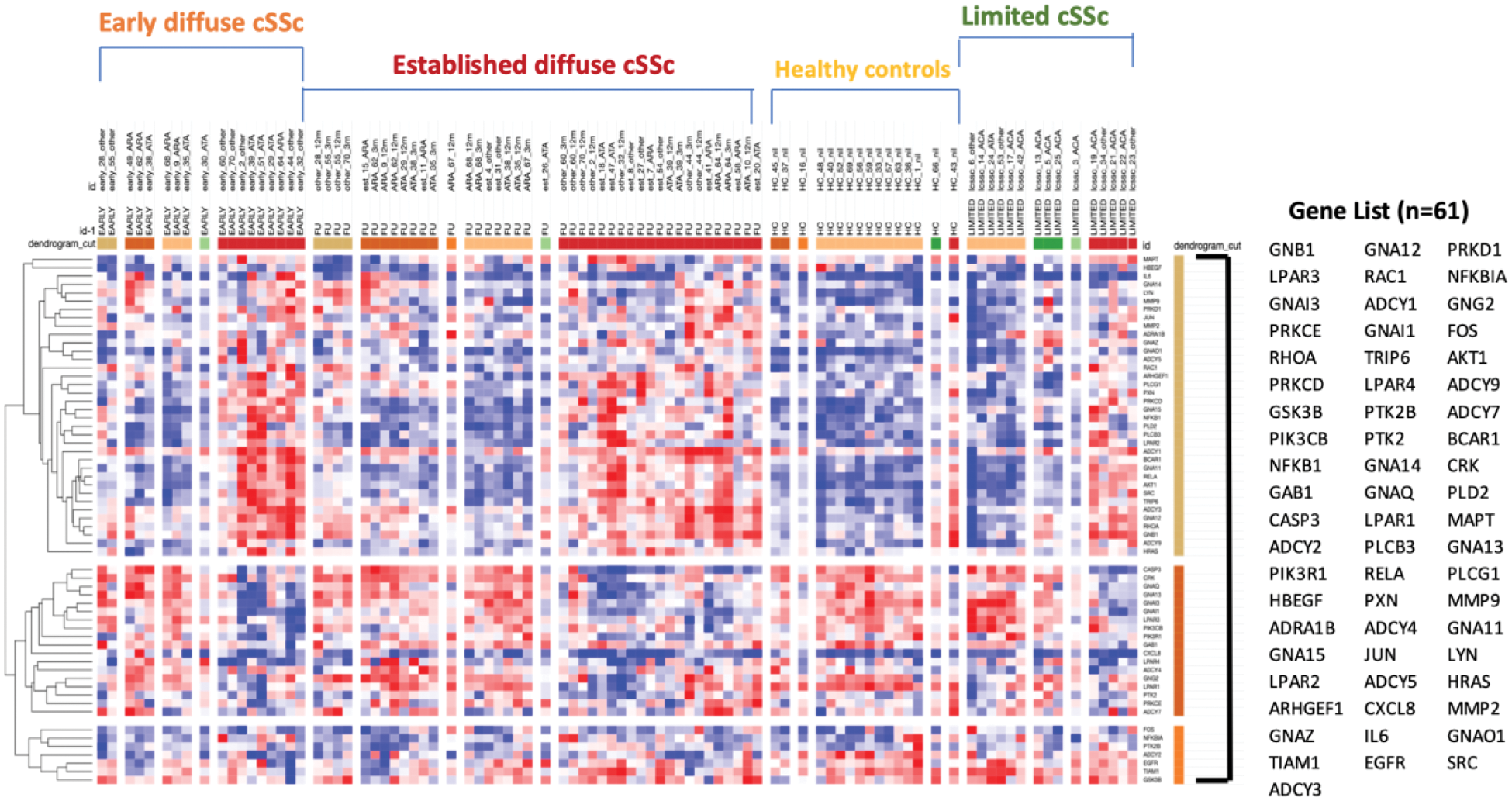
Significance analysis of microarrays showing supervised hierarchical clustering by differentially activated LPA genes and subsets of SSc and healthy controls. The LPA signature gene set is highlighted on the right and shows differential clustering across the SSc subsets. Figure generated in Morpheus software by the Broad institute.
The greatest differential gene expression of LPA regulated genes across the diffuse SSc subsets (early, established and follow up diffuse SSc) showing statistical significance compared to healthy controls. Adjusted p value (<0.05) and fold change is calculated for each gene
| Gene | adjusted p value | FC |
|---|---|---|
| GNB1 | 0.001143907 | 1.18088779 |
| LPAR3 | 0.006315113 | 1.14756639 |
| GNAI3 | 0.003318129 | 1.16192829 |
| RHOA | 0.001075626 | 1.18233583 |
| PRKCD | 0.000559052 | 1.19136157 |
| NFKB1 | 0.001050577 | 1.18071521 |
| ADCY2 | 0.037502239 | 1.11353847 |
| PIK3R1 | 0.00451478 | 1.1574943 |
| GNA12 | 0.000387076 | 1.1978318 |
| RAC1 | 0.001209535 | 1.17940688 |
| ADCY1 | 0.000533424 | 1.1943321 |
| GNAI1 | 0.009898276 | 1.14195124 |
| TRIP6 | 0.00078364 | 1.18619123 |
| GNA14 | 0.000350794 | 1.19771811 |
| LPAR1 | 0.014486234 | 1.13417392 |
| PLCB3 | 0.000814814 | 1.18537453 |
| RELA | 0.000498025 | 1.19413163 |
| PRKD1 | 0.000509761 | 1.1910523 |
| BCAR1 | 0.00020788 | 1.20890194 |
| PLCG1 | 0.001067479 | 1.18068993 |
| MMP9 | 3.36047E-05 | 1.26957648 |
| GNA11 | 0.000883337 | 1.18436531 |
| GNA15 | 0.000510709 | 1.19418449 |
| LPAR2 | 0.000938978 | 1.18111537 |
| ARHGEF1 | 0.001011979 | 1.18139005 |
| GNAZ | 0.000544073 | 1.19346841 |
| TIAM1 | 0.00421682 | 1.15784747 |
| ADCY5 | 4.7735E-05 | 1.23275858 |
| IL6 | 0.00047806 | 1.36061576 |
| LYN | 0.000156833 | 1.21509168 |
| MMP2 | 0.000663122 | 1.19333289 |
| GNAO1 | 5.6714E-05 | 1.22767667 |
| SRC | 0.000928827 | 1.1826748 |
| ADCY3 | 0.00022162 | 1.20607679 |
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Medha Kanitkar: None declared, Philip Yee: None declared, Stefano Rodolfi: None declared, Voon H Ong: None declared, Christopher Denton Boehringer Ingelheim, Corbus, Janssen, Abbvie, Janssen, GlaxoSmithKline, Bayer, Sanofi-Aventis, Galapagos, Inventiva, Boehringer Ingelheim, Roche, CSL Behring, Corbus, Acceleron, Horizon, Arxx Therapeutics, Lilly, Novartis, Certa, Zurabio, Abbvie, Arxx Therapeutics, Horizon, GlaxoSmithKline, CSL Behring, Servier;
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (