

Background: Rheumatoid arthritis (RA) is a chronic and destructive inflammatory autoimmune disease predominantly affecting the joints. RA synovial fibroblasts (SFs) produce various matrix metalloproteinases (MMPs), cytokines, and chemokines. Both genetic and environmental factors contribute to the pathogenesis of RA. It has been suggested that epigenetic mechanisms also are associated with the pathogenesis of RA. Histone lysine methylation has been shown to play an important role in the activation of RASFs. We have demonstrated that histone lysine methyltransferases (HKMTs), including myeloid/lymphoid or mixed-lineage leukemia 1 (MLL1) and MLL3, are aberrantly expressed in RASFs [1]. MLL1 and MLL3 catalyze the methylation of histone H3 lysine 4 (H3K4) and are each part of complex proteins associated with Set1 (COMPASS)-like complexes, which include WD repeat domain 5 (WDR5) [2]. WDR5 is essential for the methylation of H3K4 by MLL1 and MLL3.
Objectives: MLL1 and MLL3 are suggested to be involved in the pathogenesis of RA. The aim of our study was to determine the epigenetic mechanism by which MLL1 and MLL3 activate RASFs.
Methods: Human SFs were isolated from synovial tissues derived from RA or osteoarthritis (OA) patients undergoing total knee joint arthroplasty. MLL1 and MLL3 mRNA levels of above SFs were determined after tumor necrosis factor α (TNFα) stimulation. Trimethylation of H3K4 (H3K4me3) is present in the promoters of actively transcribed genes. We investigated changes in mRNA levels of RA-associated genes (matrix-degrading enzymes, cytokines, and chemokines) and H3K4me3 levels in the promoters of those genes on small interfering RNA-mediated depletion of MLL1 and MLL3 in RASFs. We then determined the levels of H3K4me3 and mRNAs following treatment with MM-102, which is a WDR5 inhibitor. H3K4me3 levels in the gene promoters were also compared between RASFs and OASFs. Differences between unpaired or paired groups were evaluated using the Mann–Whitney U test or the Wilcoxon signed-rank test, respectively.
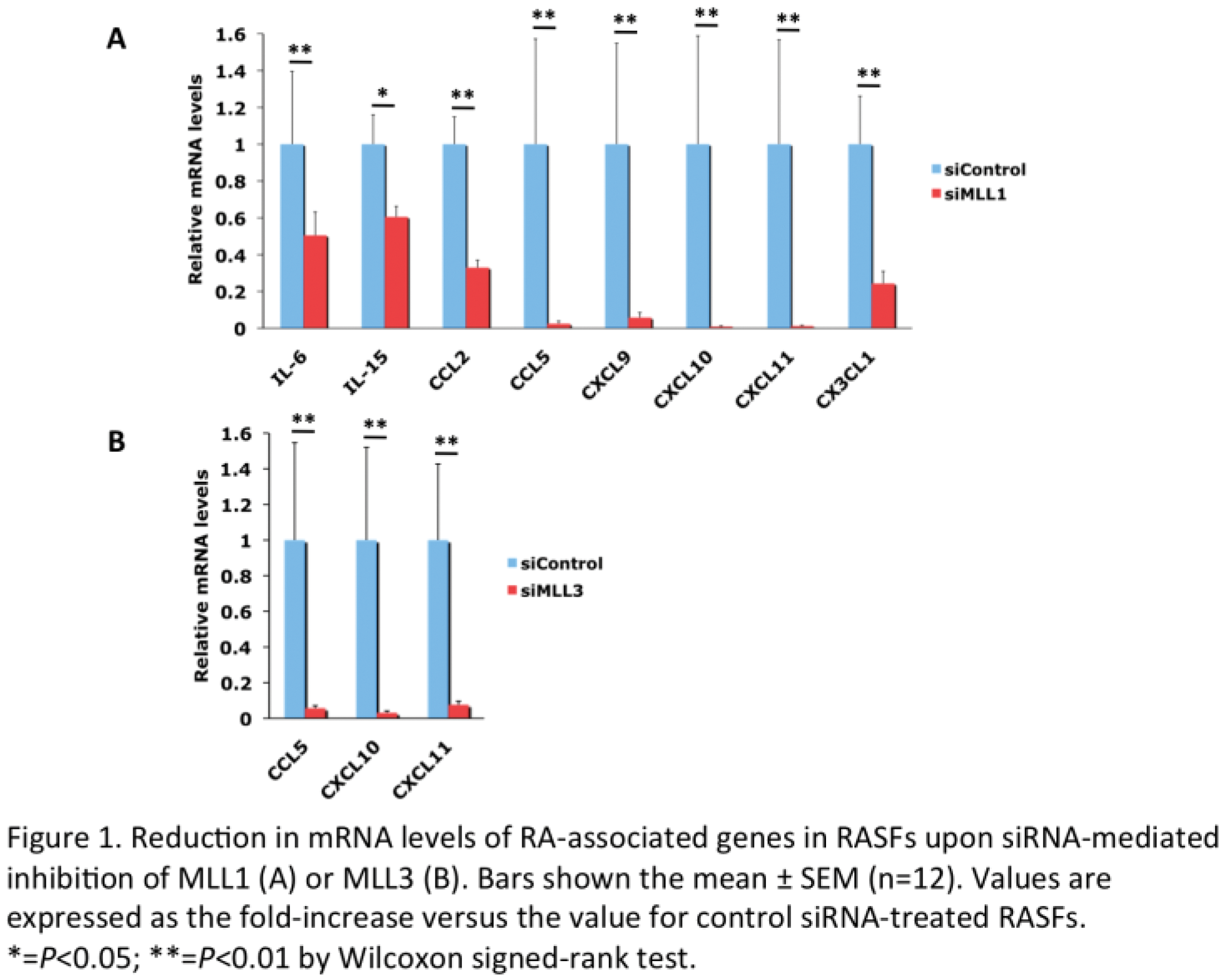
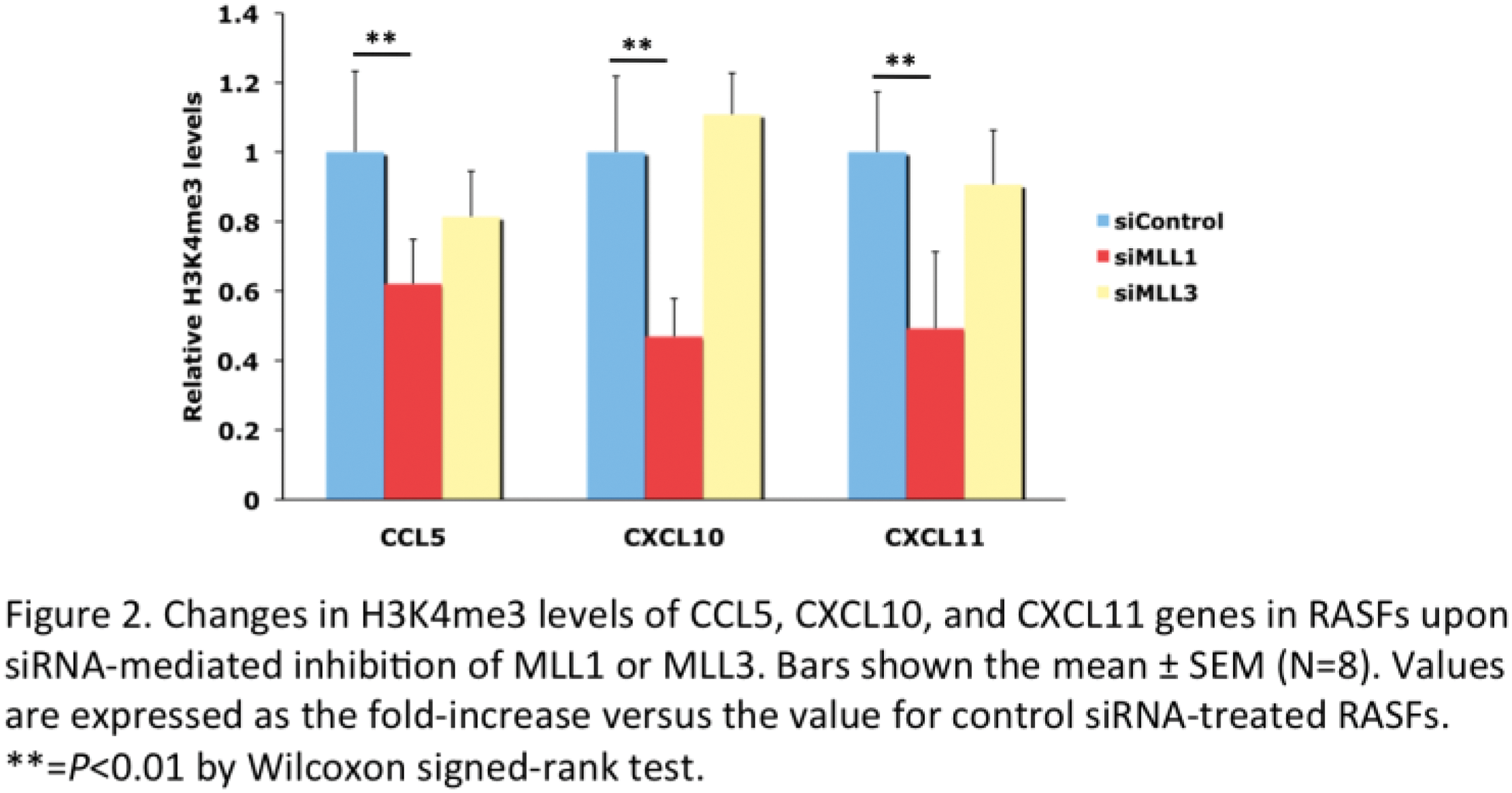
Results: MLL1 and MLL3 mRNA levels were significantly higher in RASFs than OASFs after stimulation with TNFα. MLL1 depletion significantly decreased mRNA levels of several cytokine (IL-6 and IL-15) and chemokine (CCL2, CCL5, CXCL9, CXCL10, CXCL11, and CX3CL1) genes in RASFs (Figure 1A). Correspondingly, MLL1 depletion significantly reduced H3K4me3 levels in the promoters of the cytokine and chemokine genes in RASFs (Figure 2). These results indicate that MLL1 activates cytokine and chemokine genes by the increase in the promoter H3K4me3 levels in RASFs. Interestingly, MLL3 depletion also suppressed mRNA levels of CCL5, CXCL10, and CXCL11 genes in RASFs (Figure 1B). However, H3K4me3 levels in the promoters of the chemokine genes were not altered by MLL3 depletion in RASFs (Figure 2). Since it has been reported that MLL3 is involved in the generation of monomethylation of H3K4 (H3K4me1) in the enhancers of actively transcribed genes [3], our results suggest that MLL3 may activate CCL5, CXCL10, and CXCL11 genes by the increase in the enhancer H3K4me1 levels. We plan to analyze histone methylation in the enhancers of the chemokine genes in RASFs in the future. MM-102 significantly decreased the promoter H3K4me3 and mRNA levels of the CCL5, CXCL9, CXCL10, and CXCL11 genes in RASFs. H3K4me3 levels in the promoters of the IL-6, IL-15, CCL2, CCL5, CXCL9, CXCL10, CXCL11, and CX3CL1 genes were significantly higher in RASFs than OASFs.
Conclusion: This study elucidated the epigenetic mechanism by which MLL1 and MLL3 cooperate to activate chemokine genes in RASFs. These findings may lead to new treatments like targeting histone methyltransferases for RA.
REFERENCES: [1] Araki Y et al. Altered gene expression profiles of histone lysine methyltransferases and demethylases in rheumatoid arthritis synovial fibroblasts. Clin Exp Rheumatol. 36(2):314-316. 2018.
[2] Shilatifard A. The COMPASS family of histone H3K4 methylases: Mechanisms of regulation in development and disease pathogenesis. Ann. Rev. Biochem. 81:65–95. 2012.
[3] Hu D. The MLL3/MLL4 branches of the COMPASS family function as major histone H3K4 monomethylases at enhancers. Mol Cell Biol. 33:4745–54. 2013.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (