

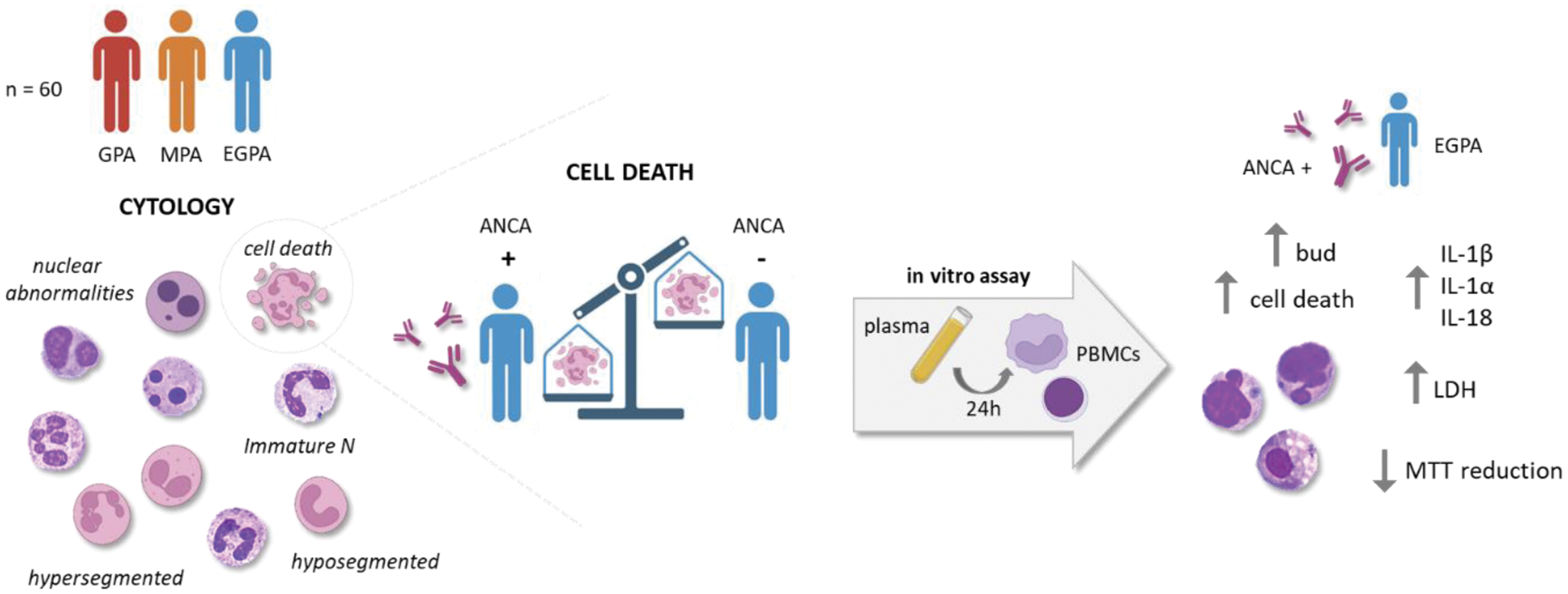
Background: In the pathogenesis of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), neutrophils (N) play a crucial role in regulating the inflammatory process. The role of monocytes (M) in AAV pathogenesis is less examined, although in vitro and in vivo evidence suggests that monocytes also play an important role in the initiation and maintenance of autoimmunity (pathologic responses to ANCA). Few studies have explored abnormalities in circulating leukocytes (nuclear abnormalities, cell death markers) in AAV patients. Recent studies suggest that some rheumatological diseases, including AAV, are associated with overall genomic instability and increased sensitivity to DNA damage. However, no data are available on leukocyte abnormalities and their relationship with inflammation and pathogenesis in AAV patients.
Objectives: Therefore, the first aim of the study was to perform a cytological evaluation of leukocytes to assess the presence of immature cells and genomic instability within three groups: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA). According to the cytological findings, we aimed at evaluating in vitro the effect of plasma from EGPA ANCA -positive and -negative patients on peripheral blood mononuclear cells (PBMCs) metabolism, cell death, and inflammatory cytokines release.
Methods: Blood samples were collected from adult patients affected by GPA (n = 17), MPA (n = 14) and EGPA (n = 29), classified according to the EULAR/ACR criteria 2022, who attended the outpatients Vasculitis Clinic of Padua University. All patients were no longer taking steroid therapy and were in the remission stage, as per the EULAR/ACR definition. May Grunwald-Giemsa staining was used to examine cellular morphology and to perform a cytogenic evaluation of leucocytes in air-dried blood smears. For in vitro assay PBMCs from healthy donors (HDs) were isolated by density-gradient centrifugation. EGPA patients selected for in vitro studies were subdivided into 3 groups: (1) EGPA ANCA-positive patients (ANCA++, n = 3), (2) EGPA ANCA-positive patients at diagnosis but negative the day of sampling (ANCA+, n = 3), (3) EGPA ANCA-negative patients (ANCA-, n = 3). PBMCs were primed with LPS (10 ng/ml) for 2 h, and then stimulated with 10% plasma from EGPA patients, from patients positive for antiphospholipid autoantibodies (IgG, controls) (n = 3) and from HDs (n = 3) for 24 h. The next day the MTT, trypan blue and LDH assay were performed and the supernatant and lysate were collected for IL-1β, IL-18 and IL-1α quantification.
Results: Immature N were higher in AAV than in HD (p<0.05). Additionally, in patients with GPA, hyposegmented N were higher than in EGPA patients (p<0.01). We found a higher percentage of hypersegmented N in EGPA and MPA patients than in HD (p<0.05). The preparations were examined also for micronuclei (MNi) and vacuoles. We found a higher percentage of N with vacuoles in EGPA patients than in HD (p<0.05). N with MNi were observed only in 8 GPA and EGPA patients, while MNi were absent in the N of HDs. We found a higher percentage of cell death in AAV patients than in HD (p<0.05). Interestingly, the percentage of cell death was higher in EGPA ANCA-positive patients than in EGPA ANCA-negative patients (p<0.05). In vitro studies conducted to further investigate these differences showed that plasma from EGPA ANCA++ patients induces higher PBMCs cell death than plasma from EGPA ANCA- patients, from patients positive to antiphospholipid autoantibodies and from HDs (p<0.01). Furthermore, the cell death rate in PBMCs treated with ANCA+ plasma was higher than in PBMCs treated with ANCA- plasma. Nuclear abnormalities (NA) were higher in PBMCs treated with ANCA++ plasma than in PBMCs treated with plasma from ANCA-, IgG patients and HDs (p<0.05). NA were also higher in PBMCs treated with plasma from EGPA ANCA+ patients than in PBMCs treated with plasma from HDs (p<0.05). Although not significant, MTT reduction was lower in PBMCs treated with plasma from ANCA-, IgG patients and HDs, than in cells stimulated with ANCA++ plasma and extracellular LDH levels appear to be higher in PBMCs treated with plasma from ANCA++ patients than in other groups. Finally, IL-1β, IL-18 and IL-1α release were higher in PBMCs treated with plasma from EGPA ANCA++ patients than in cells treated with plasma from EGPA ANCA-, IgG patients and HDs.
Conclusion: Patients with AAV exhibit statistically significant increase in the number of N, particularly in the count of those hyposegmented. A deeper knowledge of different abnormal cell subsets and their function could lead to a better understanding of AAV pathogenesis. Cell death rate and NA were higher in AAV patients than in HDs. Dysregulation of cell death can lead to uncontrolled or prolonged inflammatory responses. Furthermore, PBMCs treated with plasma from EGPA ANCA-positive patients exhibit an increase rate of cell death, NA and release high levels of IL-1β, IL-18 and IL-1α. These in vitro evidences suggest that uncontrolled cell death and the release of inflammatory cytokines may play an important role in AAV pathogenesis.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (