

Background: Sjögren’s syndrome (SS) is a chronic autoimmune disease primarily involving the salivary and lacrimal glands [1]. Meanwhile, endoplasmic reticulum stress (ERS) has been reported in various immune-related disorders [2], but its role—especially its interaction with IFN-γ signaling—in salivary gland epithelial cells of patients with SS has yet to be fully elucidated. Among the three classical ERS pathways (IRE1, PERK, and ATF6), the IRE1 signaling pathway has drawn considerable attention in the fields of immunity and inflammation. The AP-1 complex, comprising transcription factors such as c-Fos and c-Jun, can regulate the expression of various genes including matrix metalloproteinases (MMPs).
Objectives: This study aims to elucidate the mechanistic link between IFN-γ–induced ER stress and salivary gland epithelial damage in SS. We focus on the IRE1–JNK–AP-1 signaling axis to providing new insights and potential therapeutic targets for the prevention and treatment of SS.
Methods:
Labial salivary gland biopsies from SS patients were examined by transmission electron microscopy (TEM). Immunofluorescence staining to detect ER stress markers(GRP78/CHOP). The expression of three canonical ER stress pathways (IRE1, PERK, ATF6) were quantified.
Single-cell RNA-sequencing data from SS patient salivary glands were analyzed to identify upregulated pathways and DEGs. Circulating IFN-γ in plasma and salivary MMP9 levels were measured by ELISA.
Human salivary gland (HSG) cells were stimulated with IFN-γ.; Western blotting was detected ER stress-associated proteins (GRP78, CHOP) and phosphorylation levels of JNK, c-Fos, and c-Jun. Various inhibitors—TUDCA (an ERS inhibitor), SP600125 (a JNK inhibitor), and SR11302 (an AP-1 inhibitor)—were added to evaluate whether ERS-related proteins (GRP78, CHOP) and p-JNK, p-cFos, p-cJun phosphorylation levels decrease, and to measure changes in MMP9 secretion in the cell culture supernatants.
Results:
Transmission electron microscopy indicated marked expansion of the endoplasmic reticulum in salivary gland epithelial cells from SS patients. Western blot analysis showed increased expression of GRP78 and CHOP, with significantly elevated levels of IRE1 pathway components compared to the other ERS branches.
Single-cell sequencing analysis suggested that multiple pathways related to IFN-γ and ERS were significantly upregulated in SS patient salivary gland epithelial cells. ELISA confirmed that IFN-γ levels in plasma and MMP9 levels in saliva samples from SS patients were significantly higher than those of healthy controls.
In HSG cells stimulated by IFN-γ, ROS levels were markedly increased, and ERS markers such as GRP78 and CHOP were also elevated. Concurrently, p-JNK, p-cFos, and p-cJun phosphorylation levels were significantly higher compared to controls, indicating activation of the IRE1–JNK–AP-1 pathway. Correspondingly, MMP9 secretion in the HSG cell culture supernatant increased in a time- or dose-dependent manner following IFN-γ stimulation.
Upon addition of the ERS inhibitor, the expression levels of ERS-related proteins (GRP78, CHOP) decreased significantly. Treatment with JNK or AP-1 inhibitors led to pronounced reductions in p-JNK, p-cFos, and p-cJun phosphorylation levels, accompanied by a marked decrease in MMP9 secretion.
Conclusion: Our study provides a systematic elucidation of the potential mechanism by which IFN-γ activates the IRE1–JNK–AP-1 signaling axis to induce salivary gland epithelial cell damage. Clinical samples and single-cell sequencing data collectively reveal significant upregulation of ERS and the IFN-γ pathway in SS patients. In vitro experiments further confirm that IFN-γ stimulation increases ERS levels and activates the IRE1–JNK–AP-1 signaling axis, thereby elevating MMP9 secretion and exacerbating cellular injury. Inhibition of ERS or blockade of JNK/AP-1 markedly attenuates this injury process and reduces excessive MMP9 secretion. These findings shed new light on the pathogenic mechanisms of SS and suggest that the IRE1–JNK–AP-1 pathway may serve as a potential therapeutic target for mitigating SS-related glandular damage.
REFERENCES: [1] Rivière E, Pascaud J, Tchitchek N, et al. Salivary gland epithelial cells from patients with Sjögren’s syndrome induce B-lymphocyte survival and activation. Ann Rheum Dis . 2020;79(11):1468-1477. doi:10.1136/annrheumdis-2019-216588
[2] Zhou J, Pathak JL, Cao T, et al. CD4 T cell-secreted IFN-γ in Sjögren’s syndrome induces salivary gland epithelial cell ferroptosis. Biochim Biophys Acta Mol Basis Dis . 2024;1870(4):167121. doi:10.1016/j.bbadis.2024.167121
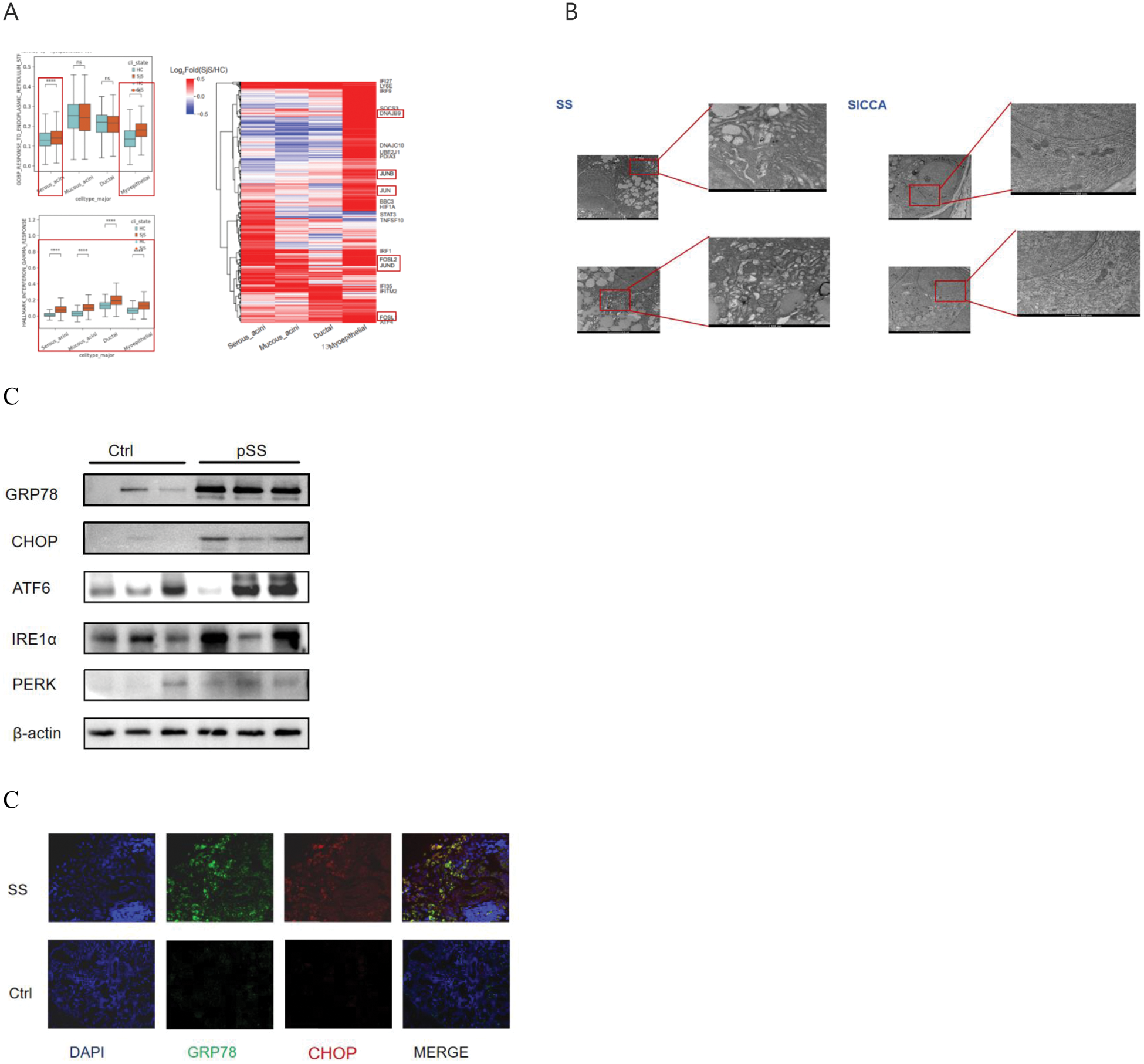
A Re-analysis of salivary gland single-cell sequencing data revealed that the IFN-γ and endoplasmic reticulum (ER) stress pathways are upregulated in salivary gland epithelial cells (SGECs). Meanwhile, differentially expressed genes in these cells showed elevated expression of IFN-γ, ER stress–related, and JNK-AP-1–related genes.
Figure B TEM of salivary gland epithelial cells from SS patients showed dilated and swollen ER lumens, with the normal lamellar folded structure completely lost.
Figure C In SS patients, ER stress markers GRP78 and CHOP were found to be upregulated in salivary gland epithelial cells. Western blot analysis of the three major ER stress pathways further demonstrated that IRE1α expression was most prominent.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (