

Background: Interleukin-1 beta (IL-1β) is an inflammasome-dependent pro-inflammatory cytokine implicated in T cell-driven autoimmune diseases [1]. To exert its function, IL-1β requires activation, typically through the cleavage of pro-IL-1β [2, 3]. Dysregulation of IL-1β has been linked to the pathogenesis of various chronic inflammatory and autoimmune diseases, including rheumatoid arthritis and juvenile idiopathic arthritis [4]. However, the precise mechanisms underlying IL-1β production in T cell-mediated autoimmunity remain unclear.
Objectives: Exploring the cellular and molecular mechanisms of IL-1β production directed by T cells for treating T cell-mediated autoimmune diseases. This study aimed to investigate whether and how activated T cells induce IL-1β production in human primary monocytes, macrophages, and dendritic cells (DCs).
Methods: Primary human monocytes, memory CD8+ T cells and memory CD4+ T cells isolated from PBMCs were used in this study. Monocytes were differentiated into GM-CSF-induced MDM and M-CSF-induced MDM by the addition of recombinant human GM-CSF or M-CSF, respectively. For DC generation, monocytes were cultured with recombinant human GM-CSF and IL-4. Monocytes/macrophages/DCs were co-cultured with memory T cells at a ratio of 1:3 and stimulated by LPS&nigericin or anti-human CD3 antibody. Small interfering RNAs (siRNA), neutralizing antibodies or inhibitors were used to study the function of genes. The knockdown efficiency was determined by quantitative PCR and western blotting. Flow cytometry, enzyme-linked immunosorbent assay (ELISA), caspase-1 activity assay and western blotting were used to quantify the production of proteins.
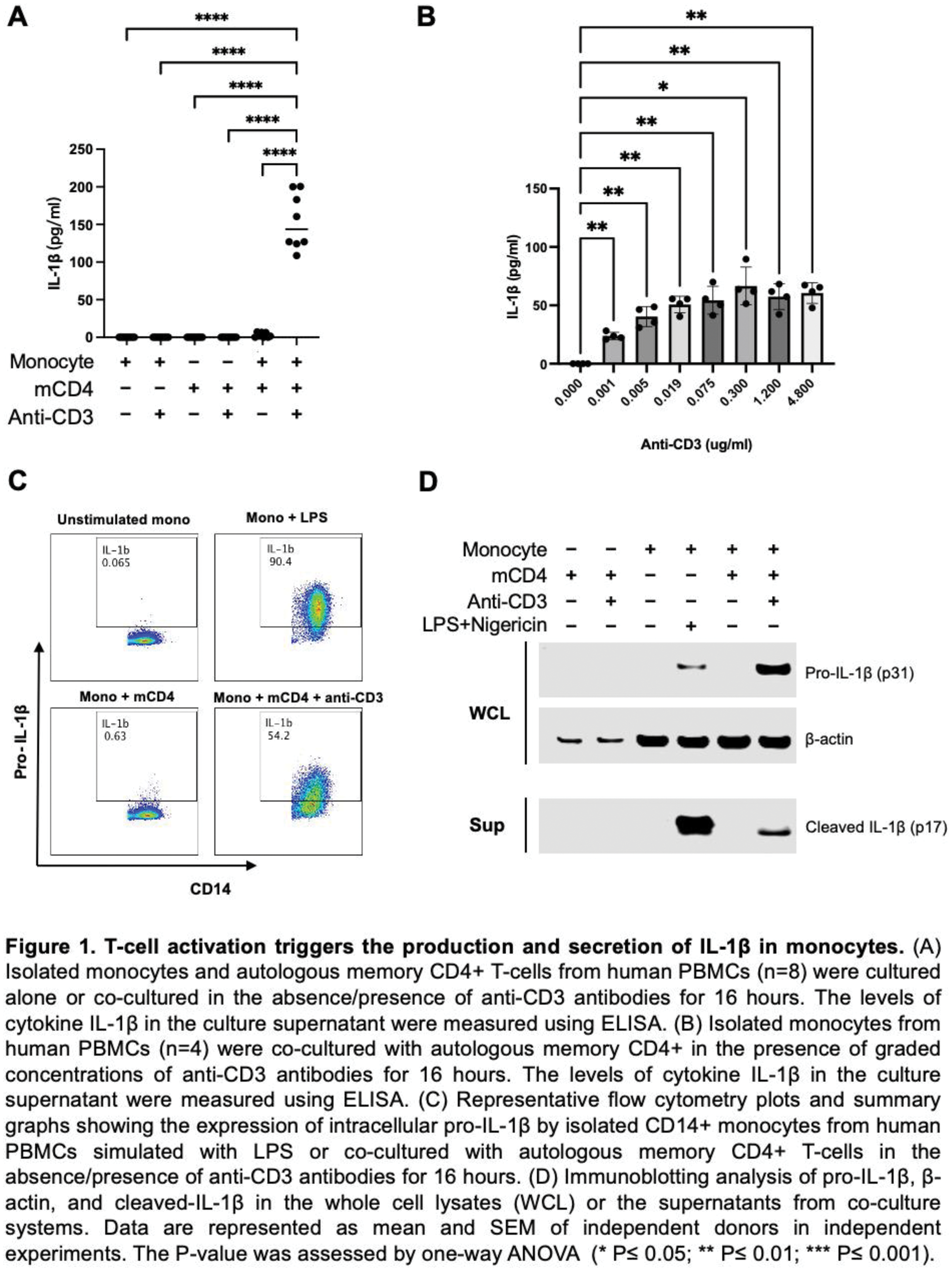
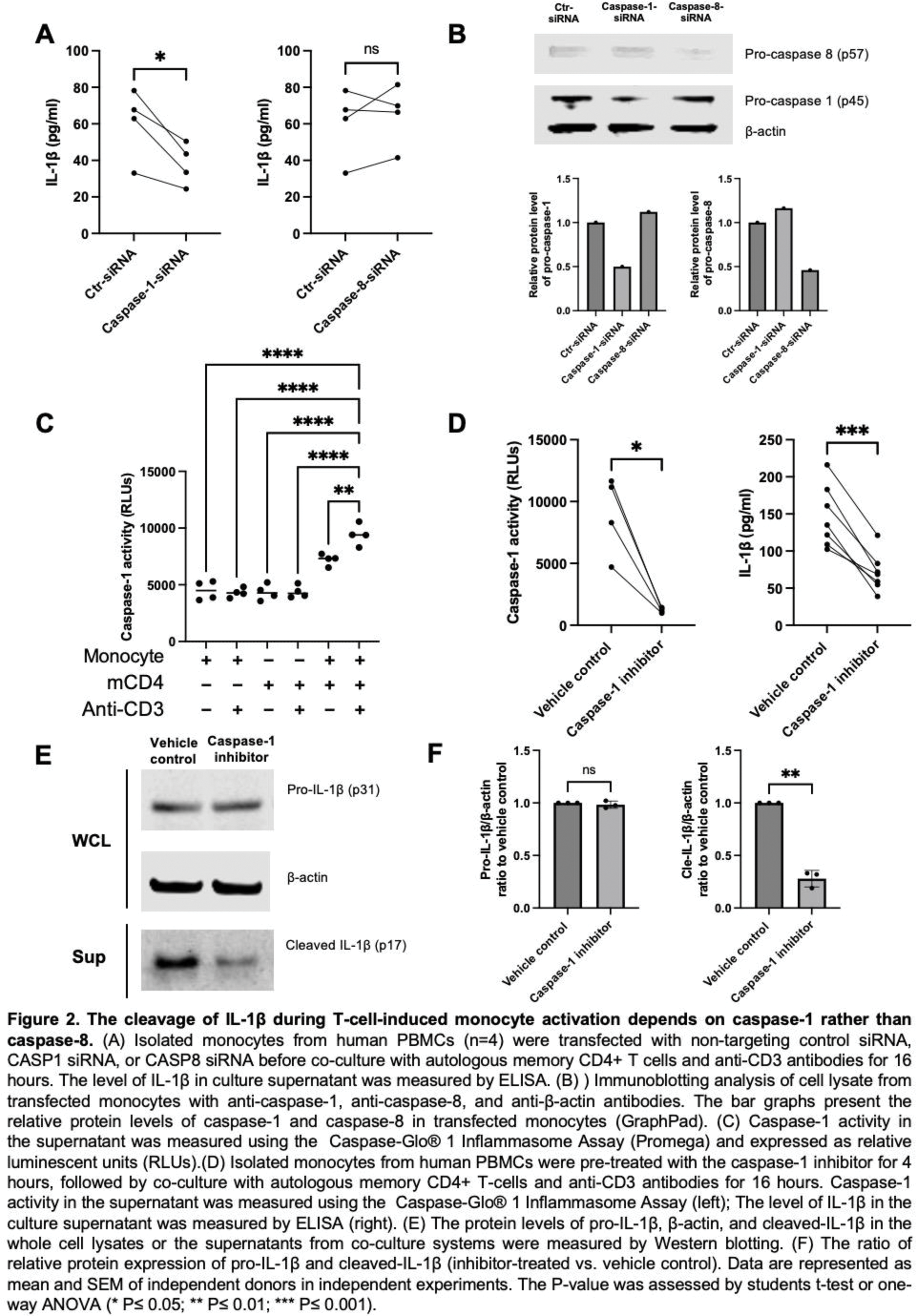
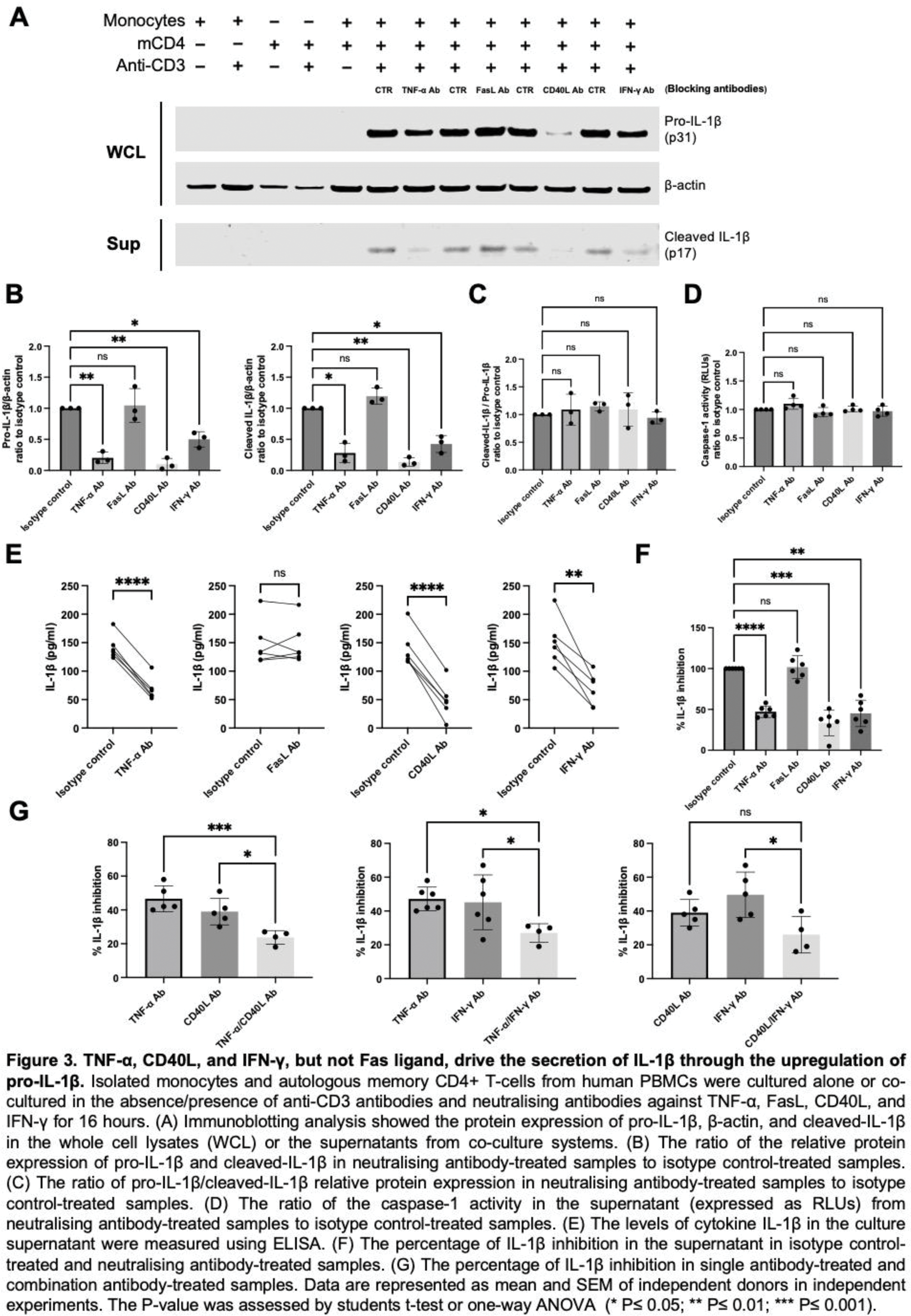
Results: Co-culturing autologous primary T cells with myeloid cells (monocytes, macrophages, and dendritic cells) revealed that both memory CD4+ and CD8+ T cells induce the expression of both pro-IL-1β and cleaved IL-1β. Antibody blocking experiments showed that the upregulation of pro-IL-1β requires the combined action of TNF-α, CD40L, and IFN-γ. Furthermore, using both siRNA and inhibitors, we found that pro-IL-1β cleavage in T-cell-instructed myeloid activation depends on caspase-1 rather than caspase-8. In addition, T-cell-induced IL-1β production is governed by shared mechanisms across different subsets of human myeloid cells.
Conclusion: Our data demonstrate that activated CD4+ and CD8+ T cells are capable of inducing IL-1β by primary human myeloid cells through the upregulation of pro-IL-1β by TNF-α, CD40L, and IFN-γ, followed by caspase-1-mediated cleavage. Our mechanistic study identifies multiple molecules upstream of this biology and offers new insights into the development of new drugs for T cell-driven autoimmune diseases.
REFERENCES: [1] Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nature Reviews Rheumatology. 2010;6(4):232-41.
[2] Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nature Reviews Immunology. 2016;16(7):407-20.
[3] Afonina Inna S, Müller C, Martin Seamus J, Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42(6):991-1004.
[4] Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12(1):14-24.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (