

Background: Rheumatoid arthritis (RA) is a chronic autoimmune disorder with predilection for synovial joints, characterized by inflammation, pain, swelling, and stiffness. Affects approximately 0.5-1% of the global population and often results in disability if left untreated [6]. RA is accompanied by the production of autoantibodies, including the Rheumatoid Factor (RF) and anti-citrullinated protein antibodies (ACPA), which play a role in disease pathogenesis and are also useful as diagnostic biomarkers [1]. RA pathogenesis is multifactorial and involves genetic, environmental, and immunological factors. Genetic markers like HLA-DRB1 alleles predispose an individual to an increased risk of developing RA, while environmental factors such as smoking have been known to precipitate disease onset [2]. Immune cells, namely T cells, B cells, macrophages, and dendritic cells, play key roles in the inflammatory process by contributing to synovial hyperplasia and joint destruction [3]. Early diagnosis and intervention are required for better outcomes to prevent permanent damage to the joints. Immune cell fingerprints in treatment-naïve RA patients and in at-risk individuals offer valuable insight into disease mechanisms and possible therapeutic targets. In this study, investigated the phenotypic composition of immune cells in treatment-naïve RA patients and their first-degree relatives, either at high risk or low risk of developing RA.
Objectives: To characterize the patterns of immune cells in peripheral blood of treatment-naïve RA patients and their first-degree relatives, high-risk ACCP+, and low-risk ACCP- individuals for the development of RA.
Methods: GSE100191 was obtained from the Gene Expression Omnibus. The following dataset consisted of 10 early active RA, 12 ACCP+, and 12 ACCP- first-degree relatives of RA patients. The CIBERSORTx computational tool was applied to estimate the abundance of 22 cell types based on gene expression data [4]. Comparisons were made using two-way ANOVA with Tukey’s correction for multiple comparisons to determine those showing significant differences from one another.
Results: We evaluate the relative percent of different immune cell populations present in peripheral blood of RA patients compared against first degree relatives with or without ACPA. The various leukocytes are further classified into neutrophils, eosinophils, mast cells, dendritic cells, macrophages (M0, M1, M2), monocytes, NK cells (resting and activated), T cells (CD4 naive, CD4 memory resting, CD4 memory activated, CD8, follicular helper, regulatory Tregs, and gamma delta), plasma cells, and B cells, naive and memory. The most significant differences in NK cell subsets were seen in the level of resting NK cells between ACCP+ relatives and RA patients. Resting NK cell levels were significantly lower in RA patients than in their ACCP+ relatives, indicating early immune dysregulation (Figure 1). Resting NK cells are effector cells in immune surveillance and regulation, and any disturbance in their levels may reflect early immunological changes before the onset of RA. In addition, it is apparent that there is variation in the remaining populations of lymphocytes, although these did attain a state of statistical significance. Since this was a consistent observation in the resting NK cell proportions in high-risk relatives and patients with RA, NK cells bear the potential to be used as an early biomarker for the risk of RA. These observations concord with previous suggestions that NK cells participate in autoimmune regulation and may be an indicator of immune imbalance [5].
Relative distribution of immune cell populations in peripheral blood samples from ACCP- relatives, ACCP+ relatives, and RA patients . The stacked bar plot represents the percentage composition of various immune cell types, including neutrophils, eosinophils, mast cells, dendritic cells, macrophages (M0, M1, M2), monocytes, natural killer (NK) cells (resting and activated), T cells (CD4 naive, CD4 memory resting, CD4 memory activated, CD8, follicular helper, regulatory Tregs, and gamma delta), plasma cells, and B cells (naive and memory). The significant difference observed was in the proportion of resting NK cells between ACCP+ relatives and RA patients, indicating possible early immunological shifts linked to RA risk.
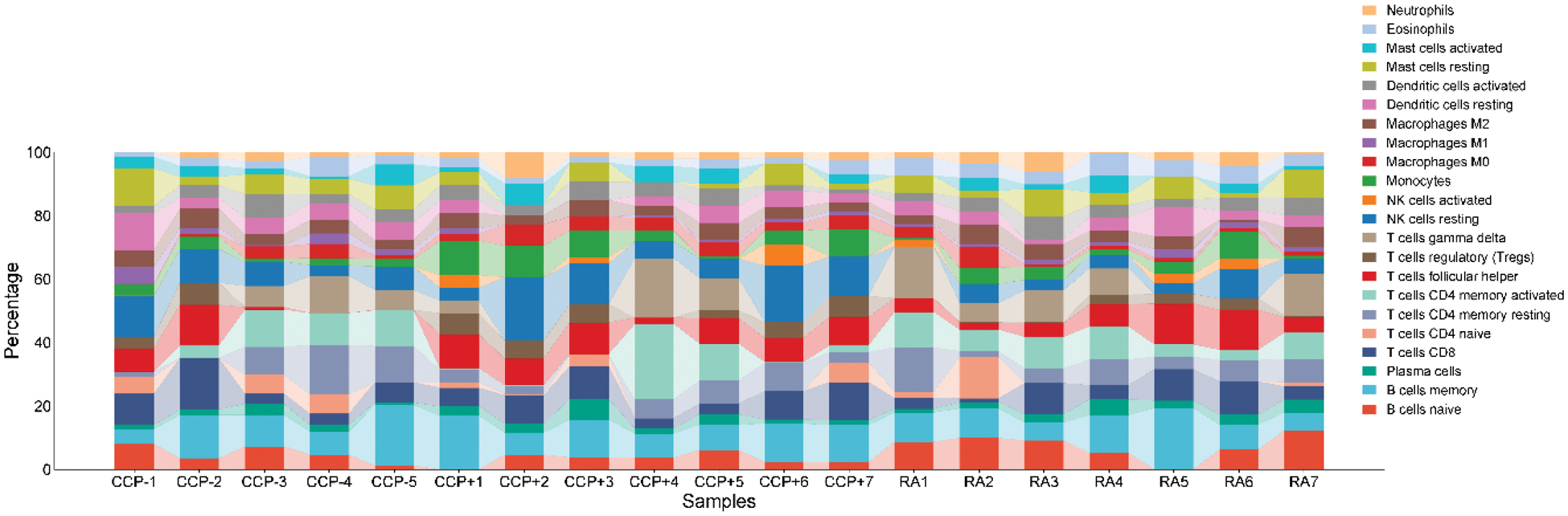
Conclusion: Except for resting NK cells, our findings suggest a lack of distinct immune cell signatures in treatment-naïve RA patients when compared to both ACCP- and ACCP+ healthy relatives of RA patients. These results highlight the importance of NK cell regulation in the early stages of RA and suggest that further studies are needed to elucidate their role in disease progression and risk assessment.
REFERENCES: [1] Aletaha, D., Neogi, T., Silman, A. J., et al. (2010). 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & Rheumatism , 62(9), 2569-2581.
[2] Klareskog, L., Padyukov, L., Lorentzen, J., & Alfredsson, L. (2006). Mechanisms of disease: genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nature Clinical Practice Rheumatology , 2(8), 425-433.
[3] McInnes, I. B., & Schett, G. (2011). The pathogenesis of rheumatoid arthritis. The New England Journal of Medicine , 365(23), 2205-2219.
[4] Newman, A. M., Liu, C. L., Green, M. R., et al. (2015). Robust enumeration of cell subsets from tissue expression profiles. Nature Methods , 12(5), 453-457.
[5] Schleinitz, N., Vely, F., Harle, J. R., & Vivier, E. (2010). Natural killer cells in human autoimmune diseases. Immunology , 131(4), 451-458.
[6] Smolen, J. S., Aletaha, D., & McInnes, I. B. (2016). Rheumatoid arthritis. The Lancet , 388(10055), 2023-2038.
Acknowledgements: We’d like to thank the Mexican National Council of Humanities, Science and Technology (CONAHCYT) for providing the academic grant that made this research possible. Grant ID: PCC-2022-320697.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (