

Background: Immune-related adverse events (irAEs) represent a distinct category of drug toxicity that affects up to 50% of cancer patients treated with immune checkpoint inhibitor (ICI) therapy within the first year of treatment [1]. Among the many biomarkers associated with irAEs, interleukin-6 (IL-6) has gained increasing prominence [2]. It remains unclear whether high or low levels of IL-6 predispose patients treated with ICIs to the development of irAEs.
Objectives: To analyze the association between IL-6 levels measured in the peripheral blood of patients with cancer before and after starting ICI therapy and the risk of developing irAEs.
Methods: This is a preliminary analysis of the AUTENTIC project, a multicenter, observational, prospective cohort designed to identify factors potentially predictive of irAEs. Informed consent was obtained from all participants before their consecutive enrollment. Inclusion criteria were: (1) initiation of treatment with one ICI or a combination of ICIs; and (2) ICI-naïve status. Exclusion criteria were: (1) life expectancy <3 months from initiation of ICI therapy; (2) any contraindication to ICIs, such as active severe autoimmune disease or poor performance status (ECOG score ≥3); (3) concurrent treatment with chemotherapy, or other specific cancer therapy; or (4) active immunosuppressive treatment, including prednisone at doses >10 mg/day. We obtained a baseline sample before the first ICI cycle (pre-ICI) and a follow-up sample just before the second ICI cycle (post-ICI). The IL-6 levels in both pre- and post-ICI samples were measured using an IL-6 Enzyme Linked Immunosorbent Assay (ELISA) kit. Patients were censored if they experienced an irAE, cancer progression or death. The primary endpoint was defined as the cumulative incidence of a first irAE of any grade during the follow-up. Quantitative and categorical variables were expressed as means with standard deviations or medians with ranges, and frequencies with percentages, respectively. To assess the association between IL-6 levels and the risk of irAEs, a univariate Cox regression model was fitted with pre- and post-ICI IL-6 levels as the exposure variable, and irAEs as the main event. Covariates such as patient age, sex, autoimmune disease prior to ICI initiation, and dual ICI therapy (ipilimumab plus nivolumab) were included as potential confounders. Other patient-related characteristics included were body mass index, primary tumor origin, baseline glomerular filtration rate, abbreviated Charlson comorbidity index, and pre- and post-ICI neutrophil-to-lymphocyte ratios. A multivariate Cox regression model was fitted including all significant variables. Finally, irAE-free survival was analyzed using the Kaplan-Meier test and compared between patients with low and high plasma IL-6 levels, defined as a cut-off of 5.6 pg/mL, using the Mantel-Cox (log-rank) test. All statistical analyses were performed using the IBM SPSS Statistics, version 30.0.0.
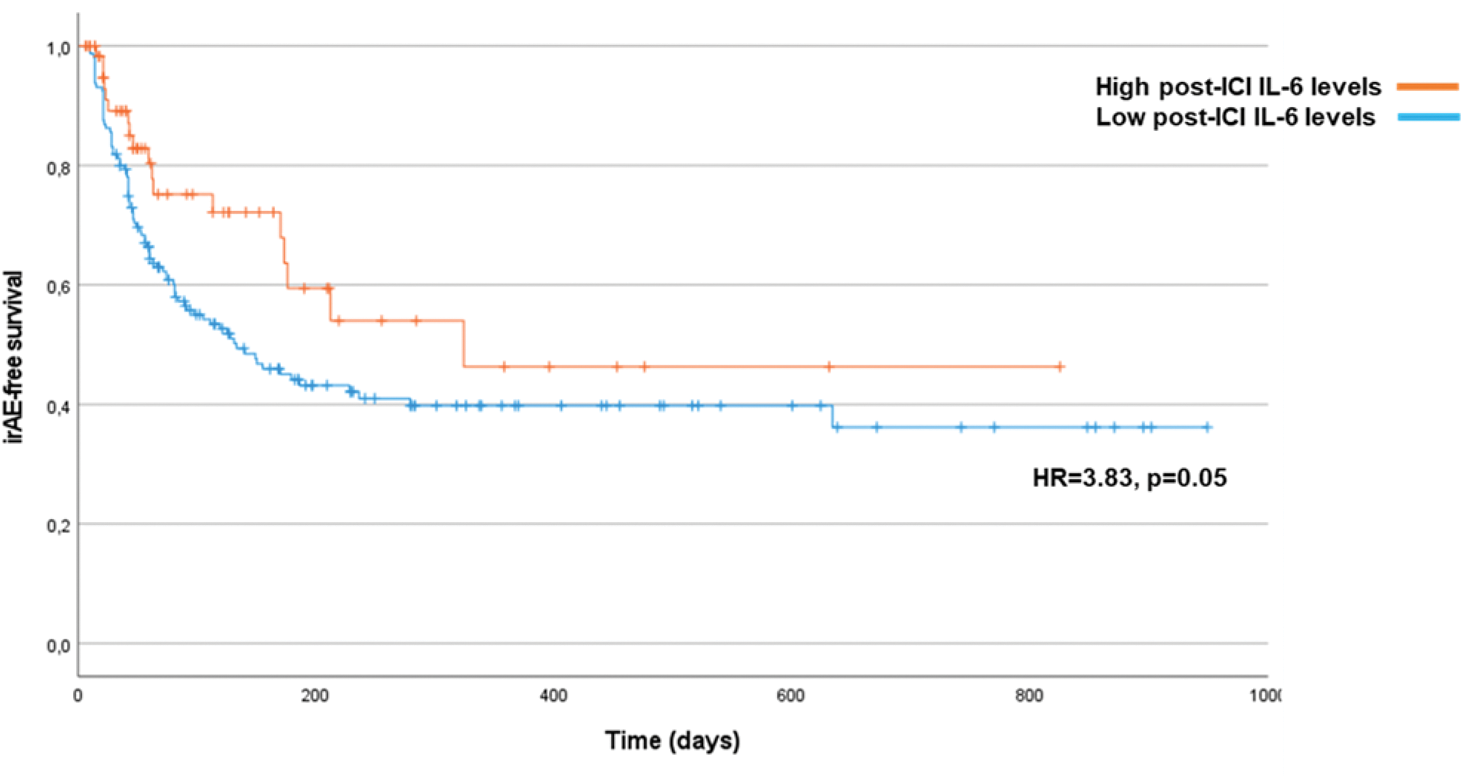
Results: Overall, 224 patients (mean age of 66.9±9.4 years, 156 men [69.6%]) were enrolled and followed up for a median of 80.5 (range 6-949) days from the time of ICI initiation. A total of 16 patients (7%) had a previous diagnosis of autoimmune disease, the most common being psoriasis (8 cases). The primary cancer was non-small-cell lung cancer in 84 cases (37.5%). During follow-up, 105 patients (47.1%) developed at least one irAE. The cumulative irAE incidence at 1 year was 46.4% (95% confidence interval [CI], 36.1-56.1%), with a median time to first irAE of 173 (95%CI=101.5-244.5) days after ICI initiation. Cutaneous and endocrinological irAEs were the most common types of first irAEs, accounting for 17 cases (32.7%) and 14 cases (26.9%) of irAEs, respectively. Univariate Cox regression model showed that female sex (hazard ratio [HR]=1.88, 95% CI=1.24-2.85, p=0.003), dual therapy with ipilimumab and nivolumab (HR=2.04, 95%CI=1.27-3.27, p=0.003), and post-ICI IL-6 levels (HR=1.05, 95%CI=1.01-1.11, p=0.039) were associated with a higher risk of developing an irAE. When these three factors (sex, dual ICI therapy, and post-ICI IL-6 levels) were included in a multivariate Cox regression model, only female sex and dual ICI therapy remained significant risk factors for irAEs (Table 1). Patients with low post-ICI IL-6 levels had a lower irAE-free survival than patients with high post-ICI IL-6 levels (Figure 1).
Conclusion: Female sex and combination therapy with ipilimumab and nivolumab are independent risk factors for irAEs, while low post-ICI IL-6 levels may also predict irAE occurrence. The observed association between low IL-6 levels and irAEs warrants reconsideration of ongoing clinical trials evaluating anti-IL-6 therapies for the prevention and treatment of irAEs [3].
REFERENCES: [1] Almutairi, A. R.; et al. Potential Immune-Related Adverse Events Associated With Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 91.
[2] Chennamadhavuni, A.; et al. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691.
[3] Weber, J. S.; et al. 1040O Phase II Trial of Ipilimumab, Nivolumab and Tocilizumab for Unresectable Metastatic Melanoma. Annals of Oncology 2021, 32, S869.
| Univariate analysis | |||
|---|---|---|---|
| Variable | HR | 95% CI | P -value |
| Female sex | 1.88 | 1.24-2.85 | 0.003 |
| Prior autoimmune disease | 1.73 | 0.96-3.11 | 0.065 |
| Dual ICI therapy | 2.04 | 1.27-3.27 | 0.003 |
| Post-ICI IL-6 levels | 1.05 | 1.01-1.11 | 0.039 |
| Multivariate analysis | |||
| Variable | HR | 95% CI | P -value |
| Female sex | 2.06 | 1.35-3.13 | <0.001 |
| Dual ICI therapy | 1.97 | 1.22-3.19 | 0.006 |
| Post-ICI IL-6 levels | 1.02 | 0.99-1.06 | 0.153 |
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: iñigo Les Bujanda This study has received a grant from the Basque and Navarra Departments of Health, David DE Haedo Sánchez: None declared, Virginia Arrazubi Arrula: None declared, Mireia Martínez-Kareaga This study has received a grant from the Basque Department of Health, Ana Campillo-Calatayud This study has received a grant from the Basque and Navarra Department of Health, Amaia Moreno Paul: None declared, Ibone DE Elejoste Echebarria: None declared, Inés Pérez-Francisco: None declared, María Cabero Zamorano: None declared, Jose Ignacio Elejalde: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (