

Background: Rheumatoid arthritis (RA) and systemic sclerosis (SSc) are both associated with increased risk of cardiovascular involvement, predominantly, atherosclerotic cardiovascular disease (CVD) and primary heart involvement respectively. Cardiovascular MRI (CMR) studies have demonstrated vascular and non-ischaemic myocardial tissue abnormalities in both diseases. We have previously undertaken proteomic study in both RA and SSc cohorts that have been characterised using CMR [1, 2]. It is unclear whether common or distinct mechanisms underlie the same cardiovascular processes.
Objectives: To identify whether common proteomic biomarkers in RA and SSc associate with vascular and myocardial tissue abnormalities and whether implicated pathways are shared.
Methods: Seventy-five patients from the Coronary Artery Disease Evaluation in Rheumatoid Arthritis (CADERA) and 78 patients from CONVAS (‘CONnective Tissue Disease and VASculitis Cohort’) and ELCASA (‘ELectrophysiology and CArdiac imaging in SclerodermA’) [1] study cohorts underwent cardiovascular magnetic resonance (CMR) imaging. Using Olink Proximity Extension Assay, normalised protein expression was measured across cardiometabolic, cardiovascular II/III and inflammation panels in patient serum samples at the time of CMR. Bayesian generalised linear regression adjusted to age, gender, systolic blood pressure and body mass index was used to identify proteins associated with CMR parameters of vascular stiffness [aortic distensibility and stiffness] and myocardial oedema/fibrosis [native T1, myocardial extracellular volume and late gadolinium enhancement]. Subsequently, an expanded protein-protein interaction (PPI) network was created using an induced network approach (String-DB) with k-means clustering applied to identify enriched functional clusters that were then subjected to KEGG enrichment analysis. Shared proteins and pathways were identified through a comparative analysis of the two cohorts.
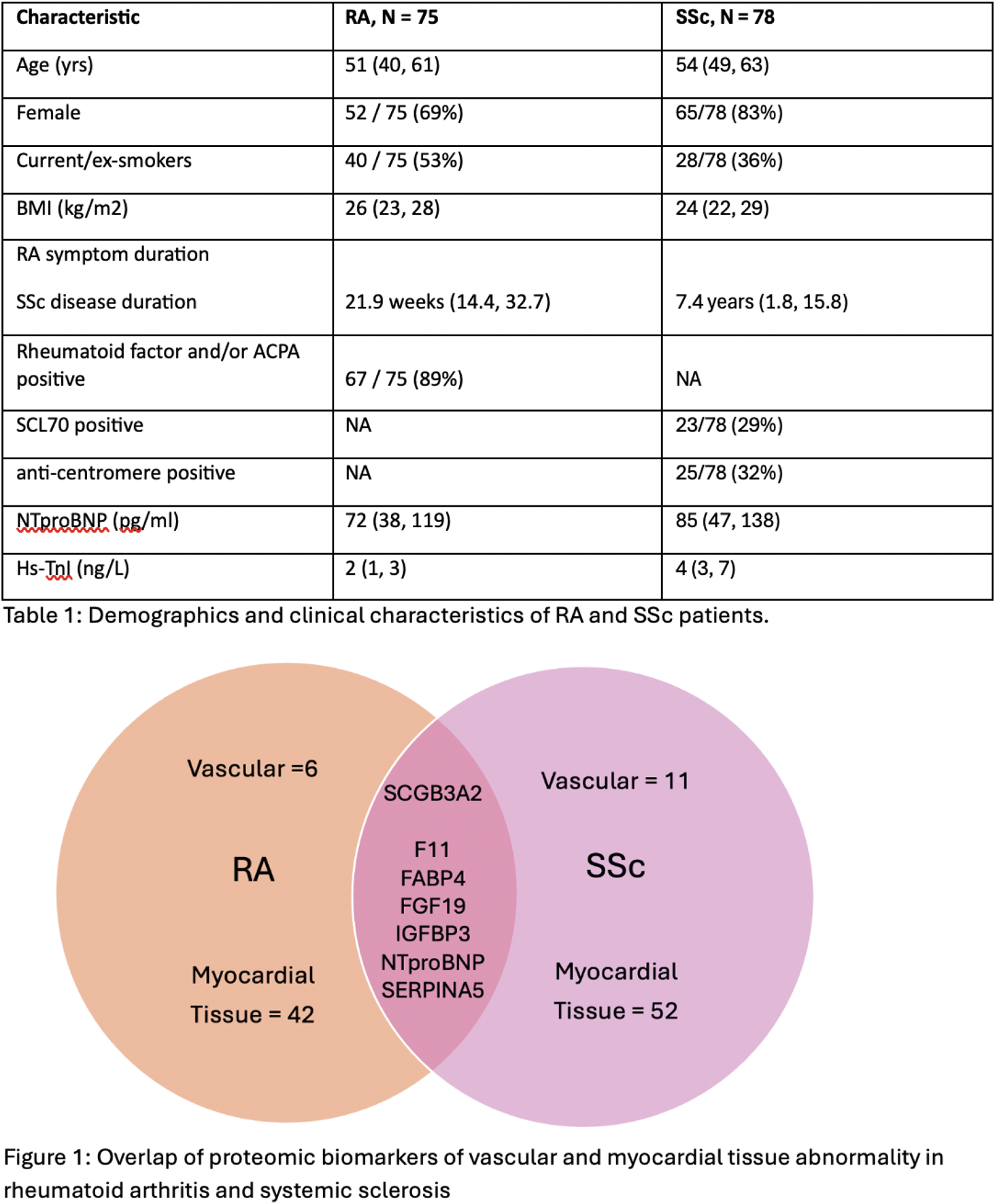
Results: Demographics and clinical characteristics of the two cohorts are presented in Table 1. Fifty-four and 70 proteins were associated with CMR measures of vascular and myocardial tissue abnormalities the RA and SSc cohorts respectively. One protein, secretoglobin family 3A member 2 (SCGB3A2) was associated with vascular abnormalities in both cohorts (aortic stiffness in RA and aortic distensibility in SSc) and 6 common proteins associated with myocardial tissue abnormalities (extracellular volume) in both cohorts – coagulation factor 11 (F11), fatty acid binding protein (FABP) 4, fibroblast growth factor (FGF) 19, insulin-like growth factor binding protein (IGFBP) 3, N-terminal pro-B natriuretic peptide (NTproBNP) and plasms serine protease inhibitor (SERPINA5) (Figure 1). Expanded PPI network analysis identified clusters involved in JAK-STAT and ErbB signalling as common pathways in both cohorts.
Conclusion: This study indicates that there may be common protein biomarkers of cardiovascular processes across two distinct rheumatic and musculoskeletal diseases, RA and SSc, and offers preliminary insights into shared mechanistic pathways and potential therapeutic targets.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Rudresh R Shukla: None declared, Amr I. Mohammed: None declared, Raluca Dumitru: None declared, Lesley Anne Bissell: None declared, Bara Erhayiem: None declared, Graham Fent: None declared, Christopher Miller CM has participated on advisory boards/consulted for AstraZeneca, Boehringer Ingelheim and Lilly Alliance, Novartis and PureTech Health, serves as an advisor for HAYA Therapeutics, has received speaker fees from AstraZeneca, Boehringer Ingelheim and Novo Nordisk, conference attendance support from AstraZeneca, and research support from Amicus Therapeutics, AstraZeneca, Guerbet Laboratories Limited, Roche and Univar Solutions B.V, Francesco Del Galdo consultancy fees and/or research support from; Abbvie, Argenx, AstraZeneca, Boehringer-Ingelheim, Capella, Calluna, Chemomab, DeepCure, Ergomed,GSK, Janssen, Mitsubishi-Tanabe, MSD, Novartis, Serono,Ventus, consultancy fees and/or research support from; Abbvie, Argenx, AstraZeneca, Boehringer-Ingelheim, Capella, Calluna, Chemomab, DeepCure, Ergomed,GSK, Janssen, Mitsubishi-Tanabe, MSD, Novartis, Serono,Ventus, Sven Plein: None declared, Darren Plant: None declared, Maya Buch Honoraria (paid to University of Manchester) and meeting support by Boehringer Ingelheim.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (