

Background: Arthritis, characterized by joint inflammation, impacts millions worldwide, with osteoarthritis (OA) being the most prevalent form. OA is a complex condition influenced by aging, obesity, mechanical stress, and genetic factors. It leads to progressive cartilage degradation, joint pain, and abnormal bone remodelling. Among the key players in this process are osteoblasts, which are responsible for bone formation and remodelling. These cells operate through intricate signalling pathways that regulate skeletal integrity. Protease-activated receptor 2 (PAR2), a G-protein-coupled receptor, has emerged as a significant regulator of osteoblast activity. PAR2 affects cartilage health, bone remodelling, and the progression of OA by modulating signalling pathways critical to osteoblast differentiation and function. Notably, the influence of PAR2 appears to vary depending on the stage of osteoblast development, presenting a dynamic role in bone-cartilage interactions. Recent studies have highlighted PAR2’s potential as a therapeutic target, prompting research into its role in osteoblast lineage signalling. This could pave the way for innovative treatments for OA and related bone-cartilage disorders.
Objectives: This study aimed to illustrate the role of the PAR2 gene in osteoblast lineage within the context of osteoarthritis (OA). The primary focus was to identify signalling pathways modulated by PAR2 and understand their implications for bone and cartilage health. Additionally, the study explored the correlation between PAR2 regulation and aging, seeking to uncover differences across various stages of PAR2 expression. It was hypothesized that inhibiting PAR2 could reduce the activity of genes associated with OA progression, offering a potential therapeutic avenue for managing the disease.
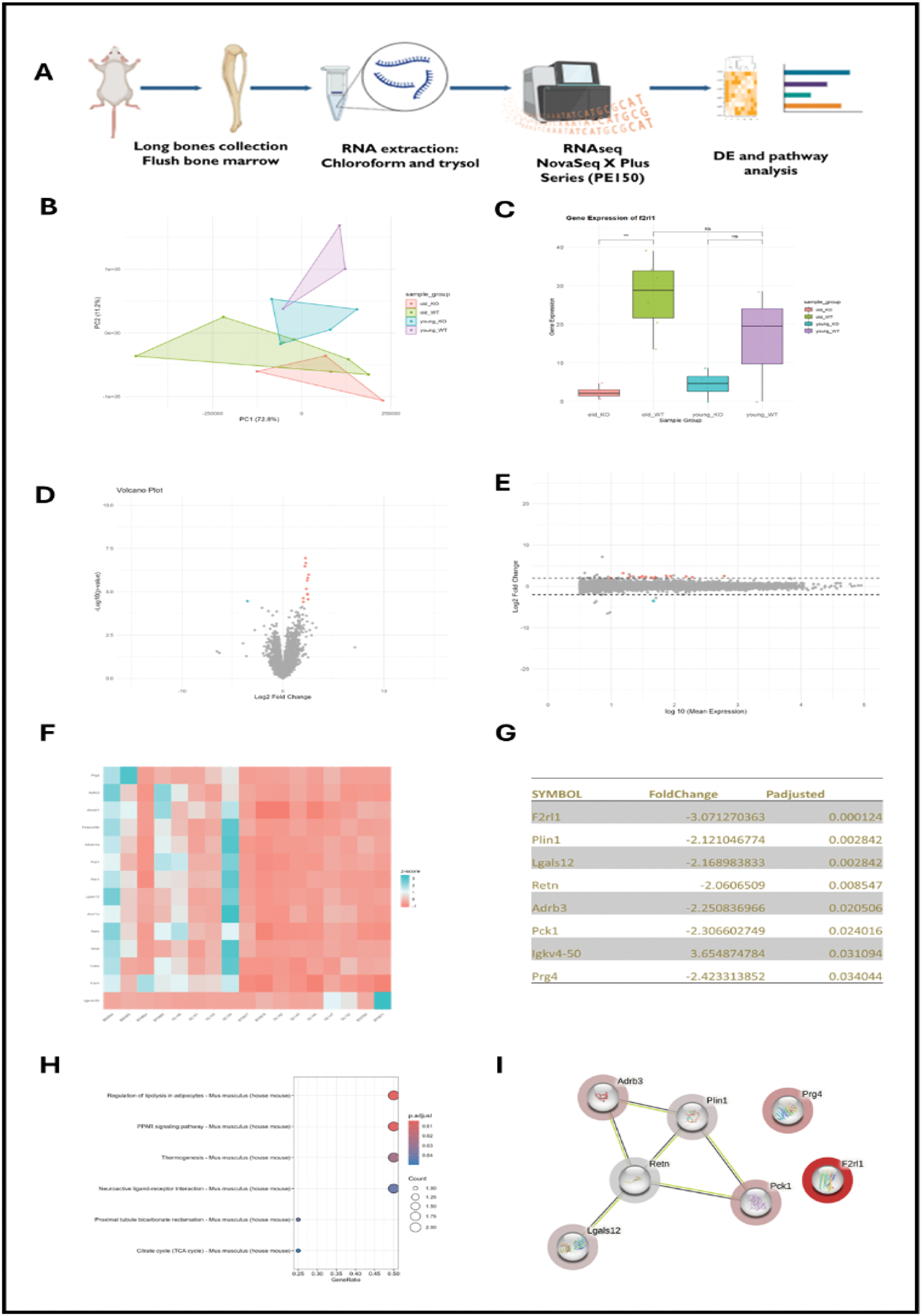
Methods: A mouse model was employed, including wild-type (WT) and PAR2-knockout (PAR2−/−) mice, with analyses focusing on their long leg bones which were separated from bone marrow. RNA was extracted from these bones and subjected to RNA sequencing to profile gene expression. Differential expression analysis was performed to identify genes and signalling pathways impacted by PAR2 regulation. To ensure the reliability of the RNA sequencing results, key findings were validated through quantitative polymerase chain reaction (qPCR). This comprehensive approach provided insights into the molecular mechanisms by which PAR2 influences osteoblast activity and OA progression.
Results: Principal Component Analysis (PCA) revealed distinct clustering patterns between wild-type (WT) and PAR2-knockout (PAR2−/−) sample groups, with clustering influenced by age. Four distinct clusters emerged, corresponding to the young WT, young PAR2−/−, old WT, and old PAR2−/− groups. Notably, a significant difference in PAR2 expression was observed between the old WT and old PAR2−/− groups, while no significant difference was detected between the young WT and young PAR2−/− groups. These findings highlight an age-dependent variation in PAR2 expression and its potential implications for osteoblast lineage and related signalling pathways. Differential expression analysis identified 15 genes impacted by PAR2 inhibition, with 14 genes being downregulated and one gene upregulated. Pathway analysis of the downregulated genes revealed the “Regulation of Lipolysis in Adipocytes” and “PPAR Signalling Pathway” as the most significantly affected pathways. A tight network of interactions was identified among key downregulated genes, including Plin1 , Adrb3 , Retn , Pck1 , and Lgals . These genes are strongly associated with gluconeogenesis and lipolysis, suggesting that PAR2 may play a critical role in metabolic processes within osteoblasts. The regulation of these pathways underscores the broader influence of PAR2 on energy metabolism and its potential link to osteoblast function and osteoarthritis progression.
Conclusion: The findings of this study underscore the pivotal role of PAR2 in regulating osteoblast function and its involvement in osteoarthritis (OA) progression. By modulating key metabolic pathways, such as lipolysis and glucogenesis, PAR2 affects the osteoblasts’ ability to maintain bone integrity, particularly in the context of aging. The age-dependent differences in PAR2 expression suggest that targeting this receptor could offer a promising therapeutic strategy for managing OA and associated metabolic disorders. Further research into PAR2’s regulation and its effects on osteoblast differentiation and function could provide valuable insights into novel treatments for OA and related conditions.
REFERENCES: NIL.
Acknowledgements: Carmen Huesa, Dr.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (