

Background: Protease-activated receptor 2 (PAR2) is a member of the G protein-coupled receptor family, known to be activated by serine proteases [1]. It has been established that PAR2 plays a pivotal role in the early stages of surgery-induced murine osteoarthritis (OA) [2, 3]. However, the question remains whether PAR2 inhibition can offer sustained, long-term protection against OA progression. Additionally, the specific cellular compartments that drive these pathological changes have yet to be determined.
Objectives: The primary objective of this study was to characterize OA pathology in global, chondrocyte-specific, and osteoblast-specific PAR2 knockout mice up to 12 months after OA induction. By doing so, we aimed to elucidate the role of PAR2 in the later stages of OA, when clinical presentation of pain and lack of joint mobility ensue. We also sought to identify whether PAR2 drives OA from the osteoblast or the chondrocyte compartment.
Methods: We utilised a murine model of OA, induced through surgical destabilization of the medial meniscus (DMM). PAR2 knockout mice were generated using the Cre-loxP system, allowing for specific deletion of PAR2 in chondrocytes (Col2cre) and osteoblasts (OCNcre). Mice were monitored for up to 12 months post-surgery, and assessed for cartilage damage, osteophyte formation, and subchondral bone changes. Histological analyses were performed to evaluate cartilage integrity, while immunohistochemistry and molecular techniques were used to investigate PAR2 expression and signalling pathways in different cellular compartments.
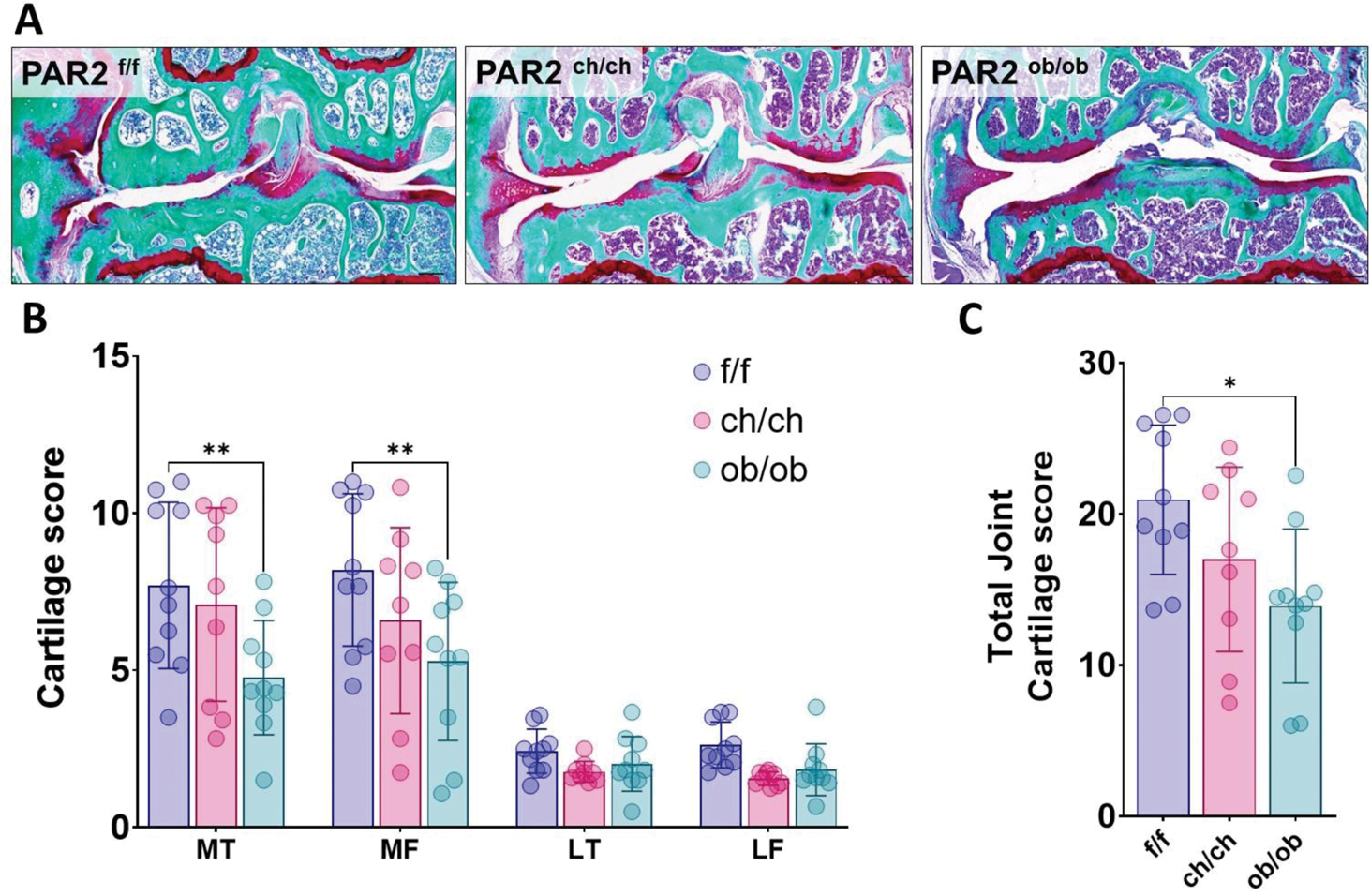
Results: Our findings revealed that wild-type mice exhibited a gradual increase in cartilage damage and loss over time, consistent with progressive OA pathology. In contrast, PAR2 knockout mice showed significantly reduced cartilage pathology, indicating a protective effect of PAR2 deletion, that was maintained with time. Notably, the removal of PAR2 specifically in osteoblasts, but not in chondrocytes, resulted in decreased cartilage damage (Figure 1). This suggests that PAR2 expression in osteoblasts plays a critical role in driving joint deterioration during the later stages of OA. Further interrogation of the osteoblast compartment revealed that PAR2 has a divergent role during osteoblast development and maturation compared to its function in already differentiated cells. Deletion of PAR2 slowed osteoblast maturation, while mineralisation was accelerated in already differentiated osteoblasts.
Conclusion: Our study highlights the importance of bone remodelling as a mechanism which influences joint degradation, where a reduction of both bone formation and resorption can lead to a slower cartilage degradation. The study also explores the complexity of PAR2-mediated effects, with age-dependent and cell-specific roles.
REFERENCES: [1] Wilkinson DJ, Arques M del C, Huesa C, et al. Serine proteinases in the turnover of the cartilage extracellular matrix in the joint: implications for therapeutics. Br J Pharmacol. 2019;176:38–51.
[2] Huesa C, Ortiz AC, Dunning L, et al. Proteinase-activated receptor 2 modulates OA-related pain, cartilage and bone pathology. Ann Rheum Dis . 2016;75:1989–97. doi: 10.1136/annrheumdis-2015-208268.
[3] Jackson MT, Moradi B, Zaki S, et al. Depletion of protease-activated receptor 2 but not protease-activated receptor 1 may confer protection against osteoarthritis in mice through extracartilaginous mechanisms. Arthritis and Rheumatology . 2014;66:3337–48. doi: 10.1002/art.38876.
. Deletion of PAR2 in osteoblasts protects against cartilage damage. (A) Representative histological sections stained with SafraninO/Fast green of wild type control PAR2 f/f (f/f), chondrocyte driven (PAR2 ch/ch , ch/ch) and osteoblast driven (PAR2 ob/ob , ob/ob) PAR2 knock out mice 12 months after induction of OA. (B) Quantification of each quadrant of the joint cartilage damage in conditional PAR2 knockouts. MT = Medial Tibial, MF = Medial Femoral, LT = Lateral tibial, LF = Lateral femoral. Data were analysed by 2-way ANOVA with Tukey correction. C) The overall joint damage expressed as the sum of damage in all joint compartments. Data were analysed by one-way ANOVA with Bonferroni correction. Tissue-specific knockouts are compared to the PAR2 f/f control. * P< 0.05 ** P< 0.01.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (