

Background: Immunoglobulin A vasculitis (IgAV) is an inflammatory disease caused by the accumulation of immune complexes of IgA in the walls of small blood vessels, primarily in children. However, it can also affect adults, who face worse outcomes. IgAV is characterized by the presence of palpable purpura and other complications that lead to a worse prognosIS such as arthralgia/arthritis, and gastrointestinal (GI) tract symptoms [1]. Nevertheless, the most severe and life-threatening clinical manifestation of IgAV is the development of nephritis. Immunoglobulin A nephropathy (IgAN) is an inflammatory disease that shares etiopathogenic mechanisms with IgAV, although IgAN is limited to the kidney. Unfortunately, the nephritis caused by IgAV (IgAVN) is indistinguishable from IgAN, and whether IgAV and IgAN are the same condition or separate entities is still a matter of debate [2]. Currently, there are no biomarkers to differentiate those IgAV patients at a higher risk of a worse prognosis, as well as biomarkers to differentiate them from those with IgAN. In this regard, since IL1B polymorphisms have previously been linked to inflammatory diseases [3] and severe renal manifestations in IgAV [4], IL1B might serve as a genetic biomarker for the prognosis of IgAV and its discrimination from IgAN.
Objectives: To determine the role of IL1B polymorphisms in the prognosis of IgAV and in the discrimination of IgAV from IgAN.
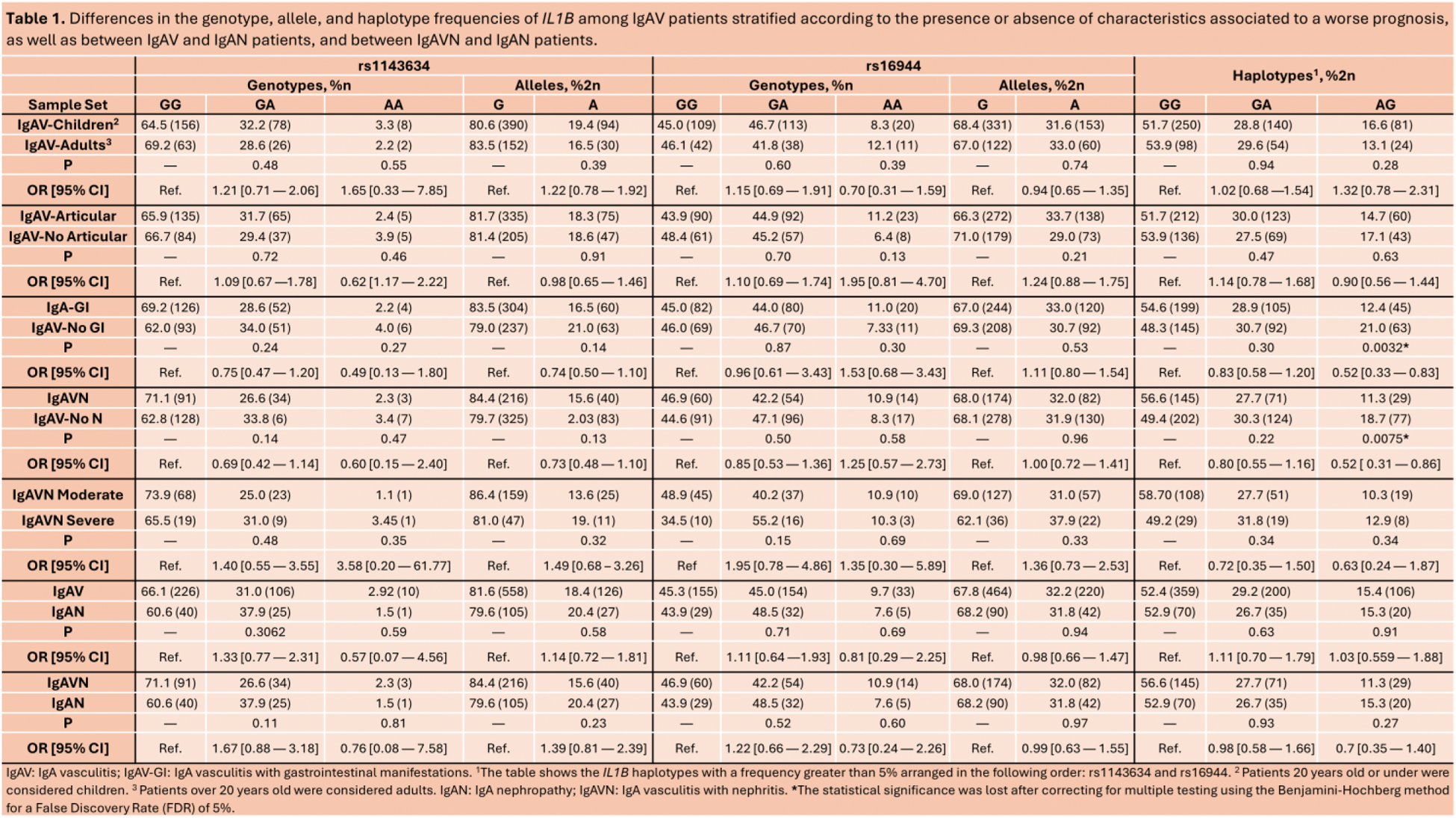
Methods: A large and well characterized cohort of 342 Caucasian IgAV patients was recruited for this study, of which 72.7% were children, 59.9% presented articular manifestations, 53.2% showed GI symptoms, and 37.5% developed IgAVN. Of the IgAVN patients, 26.9% had moderate nephritis and 8.5% had severe nephritis. Additionally, 66 Caucasian patients with IgAN were recruited to study. Two single nucleotide polymorphisms within IL1B (rs1143634 and rs16944) were genotyped using TaqMan probes. P-values ≤ 0.05, adjusted using the Benjamini-Hochberg method for a false discovery rate (FDR) of 5%, were considered statistically significant.
Results: To evaluate the role of IL1B in IgAV prognosis, we compared the IL1B genotype, allele, and haplotype frequencies in IgAV patients stratified according to the age at disease onset and the presence or absence of articular, GI, and renal manifestations, and between IgAVN patients with moderate and severe nephritis. No significant statistical differences were found between the stratified groups (Table 1). Additionally, we compared the genotype, allele, and haplotype frequencies of IL1B between IgAV and IgAN patients, and between IgAVN and IgAN patients to determine the potential of these variants as differential biomarkers between IgAV and IgAN. No statistically significant differences were observed neither between IgAV and IgAN patients or between IgAVN and IgAN patients (Table 1).
Conclusion: The IL1B polymorphisms assessed in the present study do not seem to be associated with a worse prognosis in IgAV and do not seem useful in the differentiation of IgAV from IgAN.
REFERENCES: [1] Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11.
[2] Pillebout E. IgA vasculitis and IgA nephropathy: Same disease? J Clin Med. 2021 May 25;10(11):2310.
[3] Timms AE, Crane AM, Sims AM, Cordell HJ, Bradbury LA, Abbott A, et al. The interleukin 1 gene cluster contains a major susceptibility locus for ankylosing spondylitis. Am J Hum Genet. 2004 Oct;75(4):587–95.
[4] López-Mejías R, Genre F, Remuzgo-Martínez S, Sevilla Pérez B, Castañeda S, Llorca J, et al. Interleukin 1 beta (IL1ß) rs16944 genetic variant as a genetic marker of severe renal manifestations and renal sequelae in Henoch-Schönlein purpura. Clin Exp Rheumatol. 2016;34(3 Suppl 97):S84-8.
Acknowledgements: This research was funded by European Union FEDER funds and “Fondo de Investigaciones Sanitarias” from “Instituto de Salud Carlos III” (ISCIII, Health Ministry, Spain), grant number PI18/00042, PI21/00042 and PI24/00382. JCBL is a recipient of a PFIS program fellowship from the ISCIII, co-funded by the European Social Fund (`Investing in your future´), grant number FI22/00020. RL-M is a recipient of a Miguel Servet type II program fellowship from the ISCIII, co-funded by ESF (“Investing in your future”, grant number CPII21/00004.
Disclosure of Interests: Joao Carlos Batista-Liz: None declared. María Sebastián Mora-Gil: None declared. María Teresa Leonardo Cabello: None declared. Ana Cristina Peñalba Citores: None declared. Ligia Gabrie: None declared. Rafael Gálvez-Sánchez: None declared. Luis Martín-Penagos: None declared. Paz Collado: None declared. Antonio Fernández-Nebro: None declared. Gisela Díaz-Cordoves: None declared. Luis Caminal-Montero: None declared. Ana Isabel Turrión: None declared. Esteban Rubio-Romero: None declared. Manuel León Luque: None declared. Belén Sevilla-Pérez: None declared. José Luis Callejas: None declared. Francisco Javier Narváez Garcia: None declared. Paz Floranes Elorza: None declared. Esther Vicente-Rabaneda: None declared. Juan María Blanco-Madrigal: None declared. Eva Galíndez-Agirregoikoa: None declared. Santos Castañeda: None declared. Ricardo Blanco Author Ricardo Blanco had consultation fees/participation in company-sponsored speaker´s bureau from Abbvie, Pfizer, Roche, Bristol-Myers, Lilly, Janssen, and MSD. Author Ricardo Blanco has received grants/research supports from Abbvie, MSD, and Roche, Verónica Pulito-Cueto: None declared. Raquel López-Mejías: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (