

Background: T follicular helper (Tfh) cells are crucially involved in the pathogenesis of autoimmune disorders, including Sjögren’s disease (SjD, also known as Sjögren’s syndrome), by promoting effector B cell responses. However, targeting Tfh cells remains challenging. Methotrexate (MTX), which is used as the first-line medication in the treatment of autoimmune disorders, mainly suppresses B cell responses but fails to target Tfh cells.
Objectives: We previously established experimental Sjögren’s syndrome (ESS) in mice with murine immune system (MIS), and now extended this method in a novel mouse model with human immune system (HIS). In this study, we took advantage of both mouse models and aimed to develop novel drug that suppress Tfh cell-mediated humoral autoimmunity.
Methods: Based our clinical and animal studies, we conducted drug screening both in silico and in vitro . RNA-seq analysis was used to identify the targeted pathways. Chromatin immunoprecipitation (ChIP)-PCR assay was used to analyze the transcriptional activities. Surface plasmon resonance (SPR) analysis and cellular thermal shift assay (CETSA) were used to determine the binding of small molecule and target protein. CRISPR/Cas9 was utilized to perform genetic ablation. Peripheral blood mononuclear cells (PBMCs) from healthy donors or patients with SjD were isolated for culture. C57BL/6 mice were immunized to induce MIS-ESS. NOD.Cg-PrkdcscidIL2rγtm1Wjl/SzJ (NSG) mice were engrafted with cord blood-derived CD34+ human hematopoietic stem cells (HSC) to generate HIS. HIS-ESS mouse model was established by immunisation with antigens derived from human salivary A-253 cells.
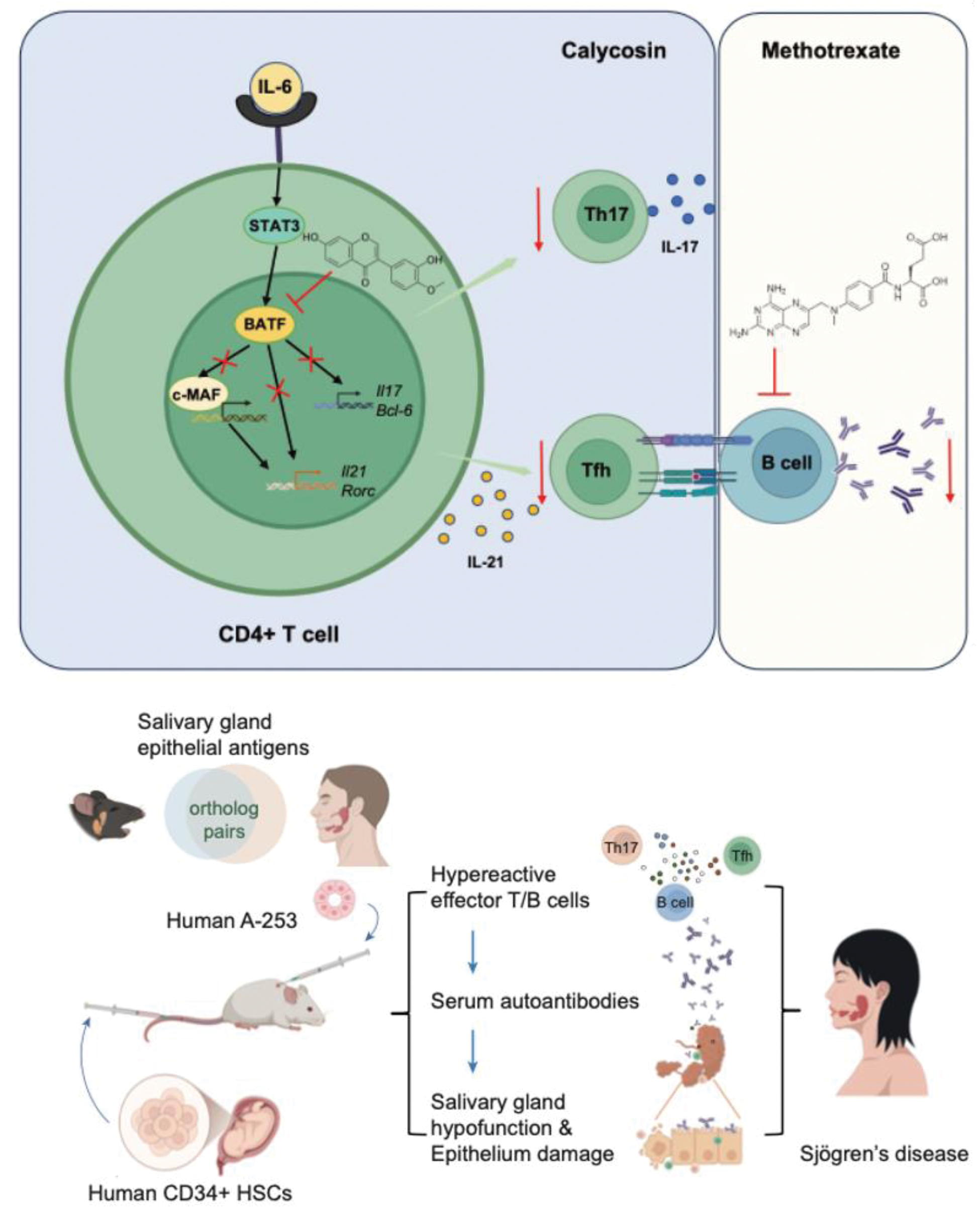
Results: We first identified that calycosin (Caly), a natural flavonoid, effectively suppressed pathogenic Tfh cell responses, although it did not affect the plasmacytic differentiation of B cells in ESS mice. Under Tfh polarization conditions, Caly rapidly bound to the master transcription factor, BATF, in both human and murine CD4+ T cells and thus potently disrupted BATF-mediated Maf gene transcription. This effect was abolished in Batf-/- T cells or c-Maf inhibitor. Our in vivo findings suggested that single treatment of either Caly or MTX was beneficial to ESS mice at acute stage, but had little therapeutic effects in mice with chronic ESS. Notably, MTX synergized with Caly and significantly attenuated the disease pathology in ESS mice with chronic inflammation, showing signs of disease remission. This synergistic effect was also supported in PBMCs from patients with SjD, and further validated in HIS-ESS mice.
Conclusion: Caly serves as a novel inhibitor of BATF in suppressing Tfh-cell-mediated humoral autoimmunity and may elicit a synergistic effect in combination with MTX or other B-cell-targeting strategies.
Upper panel: Caly, a bioactive flavonoid, binds to BATF protein, thus disrupting c-Maf transcription in human and murine CD4+ T cells. Caly potently suppressed Tfh cell differentiation and Tfh-mediated humoral autoimmunity. MTX, the first-line medication in the treatment of autoimmune disorders, but fails to target Tfh cells; Bottom panel: We established humanized mice by CD34+HSCs and induced HIS-ESS in mice with human salivary antigens. HIS-ESS mice also showed clinical features of human SjD. Notably, MTX synergizes with Caly to attenuate ESS pathology in MIS-ESS and HIS-ESS mice with chronic inflammation. Together, our findings identify Caly as a novel BATF inhibitor and potentiate Caly in the combinational treatment of humoral autoimmunity.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (