

Background: Rheumatoid arthritis (RA) is a chronic autoimmune disorder primarily affecting the joints, characterized by persistent inflammation, polyarticular pain, and progressive deformities. RA significantly increases the risk of systemic complications, including a twofold higher likelihood of myocardial infarction [1]. This heightened cardiovascular risk is attributed to accelerated atherosclerosis [2] and the formation of coronary plaques [3]. Despite advancements in understanding the individual pathophysiological mechanisms of RA and atherosclerosis, the interplay between these conditions remains poorly understood. This study employs transcriptomic data sets to uncover the molecular landscape and co-expressed pathways involved in RA-related atherosclerosis, offering insights into the shared mechanisms driving these comorbidities.
Objectives: This study aimed to analyze the molecular mechanisms of RA-related atherosclerosis and compare the immune infiltrates in the plaques of aortic biopsies of patients with RA with those without RA.
Methods: Transcriptomic datasets GSE55457 and GSE55235, containing gene expression profiles of synovial membrane samples from patients with RA, were retrieved from the Gene Expression Omnibus platform. Differentially expressed genes (DEGs) were identified using thresholds of a false discovery rate (FDR) < 0.05 and a log2 fold change > 2. Overlapping DEGs between the datasets were visualized using a Venn diagram. Co-expressed genes were predicted using the GeneMANIA online portal, and functional annotations were performed using the Metascape platform. A protein-protein interaction (PPI) network was constructed using Cytoscape software. Key hub genes (HGs) were identified using the CytoHubba plugin by applying five algorithms: Degree, DMNC, Eccentricity, Radiality, and Stress. Mean fluorescence intensities (MFIs) were extracted from dataset GSE100927, which includes gene expression profiles of 69 peripheral artery biopsies from patients with RA and healthy controls (HCs) to draw receiver operating characteristic (ROC) curves, assessing their diagnostic performance. Immune cell infiltrates in atherosclerotic aortic biopsies were analyzed using dataset GSE110008 with the CIBERSORTx online tool. The percentages of 22 immune cell types were calculated and compared between patients with RA-related atherosclerosis and those without RA. The relationship between gene expression and immune infiltration in RA-related atherosclerosis was evaluated using Spearman correlation analysis, with MFI values and immune cell percentages as variables. Statistical significance was defined as p < 0.05.
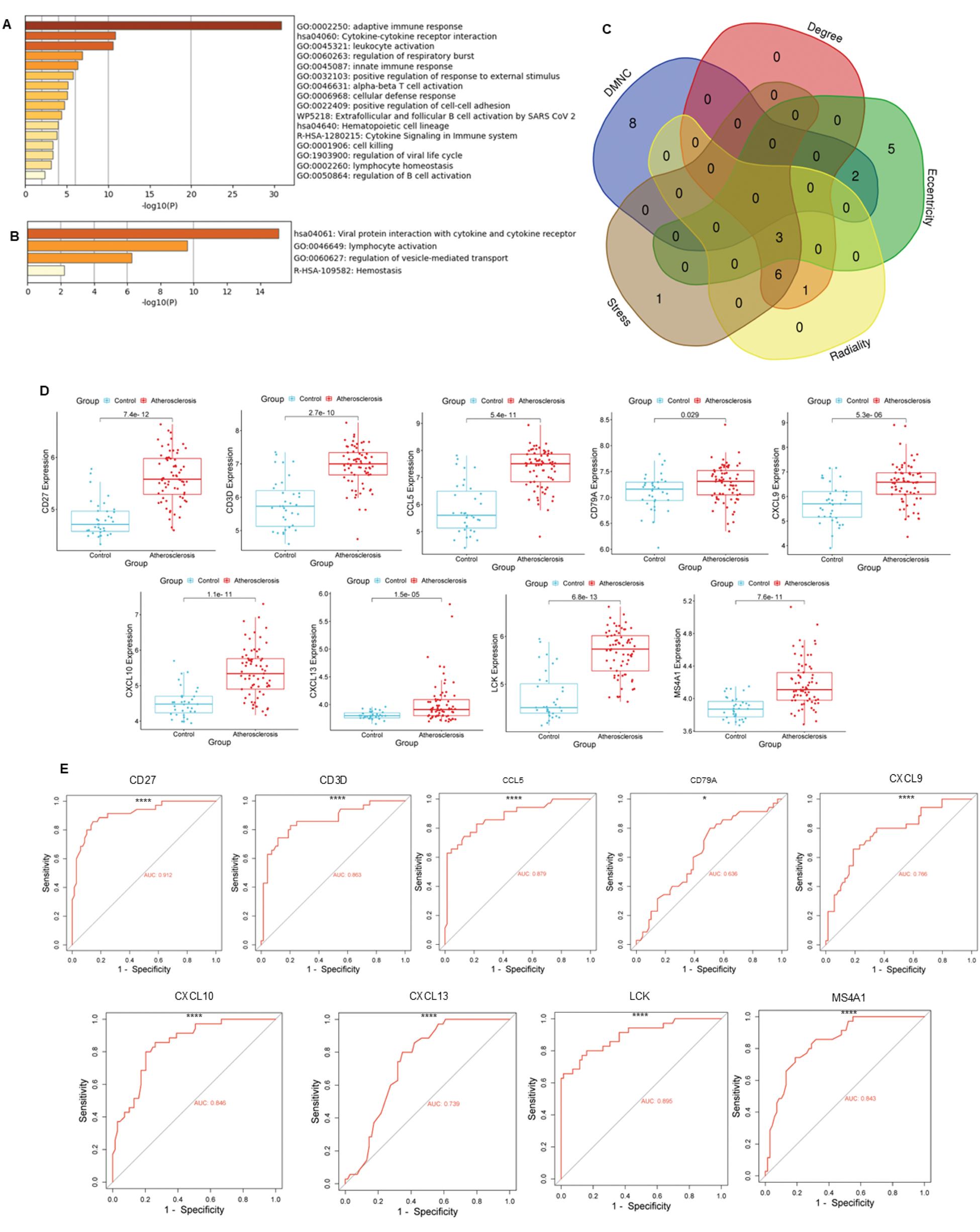
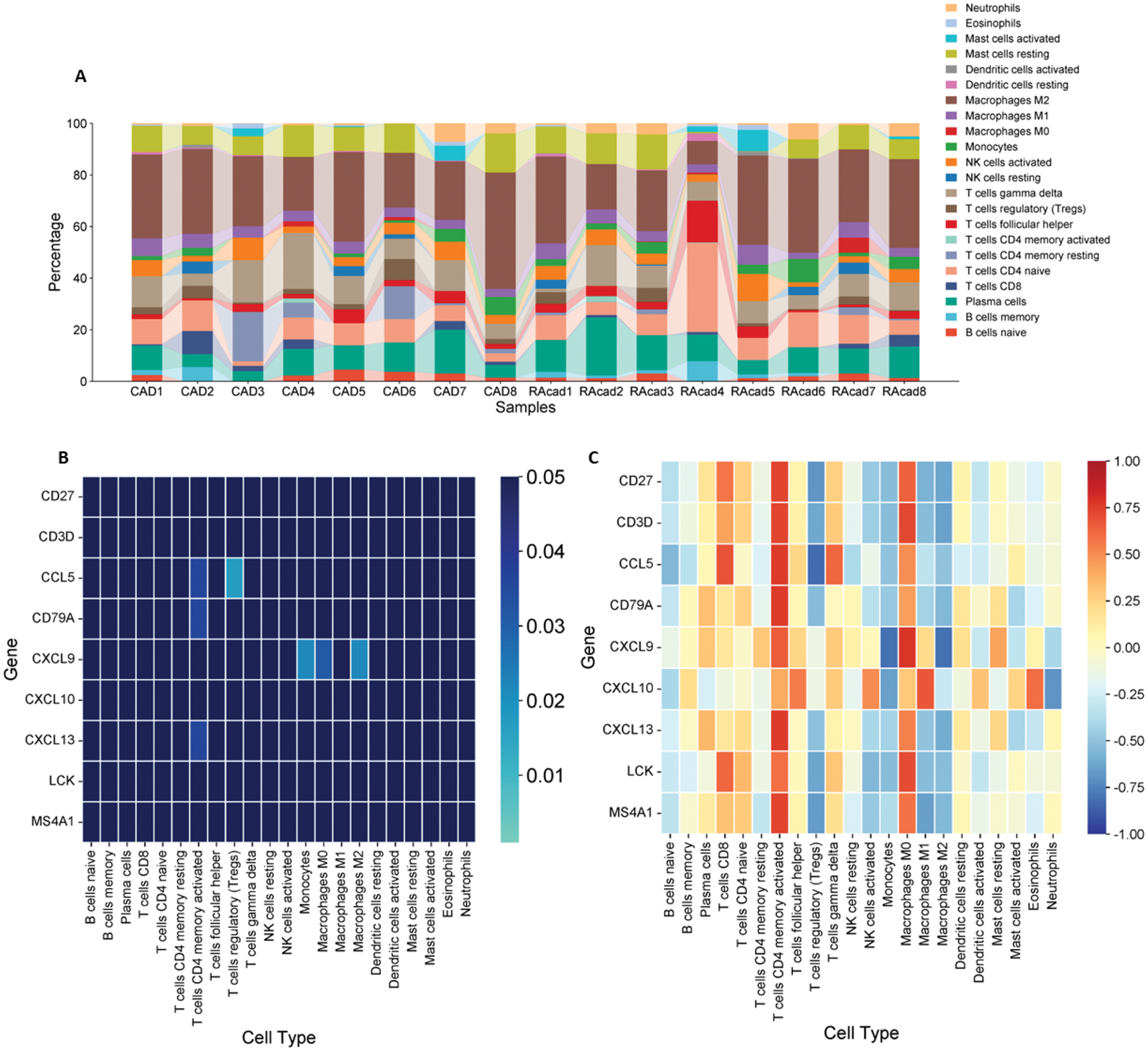
Results: 82 differentially expressed genes were filtered, related to the adaptive immune response, interactions involving cytokine receptors, and leukocyte activation (Figure 1A). 19 yielded genes were co expressed, in which viral-cytokine receptor interactions, lymphocyte activation and vesicle-mediated transport pathways were observed. 9 HGs overlapped in at least three algorithms, and their MFIs were greater in the atherosclerotic group compared to controls (Figure 1D). ROC curves displayed appropriate area under the curve (AUC) for adequate discrimination (Figure 1E). The immune infiltrate in aortic atherosclerosis of patients with RA did not differ significantly compared to those who did not have RA (Figure 2A). Conversely, in this set of patients, significant correlations were observed between CD4+ memory activated T cells with CCL5 , CD79A , and CXCL13 ; regulatory T cells with CCL5 ; and CXCL9 with monocytes, and M0 and M2 macrophages (Figure 2B and C).
DEGs, Functional Enrichment, Co-Expressed Pathways, and Diagnostic Accuracy. (A) Functional enrichment analysis of DEGs, highlighting adaptive immune response and cytokine signaling pathways. (B) Functional enrichment analysis of co-expressed genes, emphasizing immune-related and vesicle-mediated transport processes. (C) Overlap of HGs identified across five algorithms. (D) MFI values of HGs compared between atherosclerotic and non-atherosclerotic samples using Student’s t-test. (E) ROC curves illustrating the diagnostic performance of HGs, with Area Under the Curve (AUC) values provided. Significance is indicated by asterisks: *p < 0.05, ****p < 0.0001.
Immune infiltrate in aortic atherosclerotic biopsies and relationship between gene expression. (A) Percentages of immune cells infiltrated in aortic atherosclerotic samples of patients with RA (RAcad) and without RA (CAD). (B) Correlations between immune cells and gene expression. Significant correlations are shown in lighter blue, while positive correlations are depicted in red and negative in blue.
Conclusion: Key DEGs were identified in synovial membrane samples. Immune infiltrate analysis in RA and non-RA patients showed no significant differences in conventional atherosclerosis, highlighting shared pathophysiology. Significant correlations between immune infiltrate and gene expression were also found, requiring further in vivo validation.
REFERENCES: [1] Jagpal, A., & Navarro-Millán, I. (2018). Cardiovascular co-morbidity in patients with rheumatoid arthritis: A narrative review of risk factors, cardiovascular risk assessment, and treatment. BMC Rheumatology, 2 (10).
[2] Reiss, A. B., Silverman, A., Khalfan, M., & Vernice, N. A. (2019). Accelerated atherosclerosis in rheumatoid arthritis: Mechanisms and treatment. Current Pharmaceutical Design, 25 (9), 969–986.
[3] Humphreys, J. H., Warner, A., Chipping, J., Marshall, T., Lunt, M., & Symmons, D. P. (2014). Mortality trends in patients with early rheumatoid arthritis over 20 years: Results from the Norfolk Arthritis Register. Arthritis Care & Research, 66 (9), 1296–1301.
Acknowledgements: We’d like to thank the Mexican National Council of Humanities, Science and Technology (CONAHCYT) for providing the academic grant that made this research possible. Grant ID: PCC-2022-320697.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (