

Background: Behçet’s syndrome (BS) is a chronic, relapsing, systemic vasculitis disorder characterized by recurrent oral and genital ulcers, ocular inflammation, and cutaneous lesions, with the potential to involve multiple organ systems including the vasculature, joints, gastrointestinal tract, and nervous system. In arterial involvement, the aneurysm is the most common vascular lesion.
Objectives: To elucidate the cellular and molecular mechanisms underlying Behçet’s disease with aortic involvement, and to provide research methodology for future targeted therapy by conducting single-cell sequencing on the aortic tissue of patients with Behçet’s syndrome.
Methods: The study enrolled six participants—three with Behçet’s syndrome (BS) and three with atherosclerosis—from January 2023 to June 2024 at Beijing Anzhen Hospital, Capital Medical University. The BS patients met the criteria set by the International Study Group for Behçet’s Disease (ISG) and the International Criteria for Behçet’s Disease (ICBD). We conducted single-cell RNA sequencing on the aortas of these patients.
Results: We depicted the cellular landscape of aortic tissue in Behçet’s syndrome (BS) patients, retaining 53,520 high-quality cells for downstream analysis. Of these, 24,307 (45.42%) were from the BS group and 29,213 (54.58%) from the control group. Cells were divided into eight clusters (Figure 1A): Smooth muscle cells (26.97% of total, marked by ACTA2, DSTN, MYH11, IGFBP2, TPM2); Fibroblasts (21.22%, identified by COL1A2, COL1A1, FN1, DCN, C1R); Endothelial cells (14.13%, characterized by PECAM1, FABP4, VWF, FI27); T cells (13.60%, with CXCR4, TRBC2, CCL5, IL7R); Macrophages (10.68%, defined by C1QA, C1QB, C1QC); Mesenchymal cells (10.47%, with MT1M, IGFBP5); Mast cells (1.99%, signified by TPSB2, TPSAB1, CPA3, MS4A2); and T-cell-like SMCs (0.95%, distinguished by ACTA2, DSTN, CXCR4, CCL5). Compared to the control group, the BS group showed significant increases in endothelial cells, fibroblasts, and mesenchymal cells, and a significant decrease in smooth muscle cells (Figure 1B). From scRNA-seq data, we identified 247 up-regulated and 673 down-regulated DEGs in Behçet’s syndrome. The top 10 up-regulated DEGs were APOD, DCN, FBLN1, MTRNR2L12, SERPINF1, SFRP4, MMP2, C1S, MT-ATP8, and CCN5 (Figure 1C). Mapping these DEGs to cell types revealed that up-regulated DEGs were enriched in fibroblasts. Based on DEGs, we determined enriched biological pathways. The top enriched GO pathways for up-regulated DEGs included wound healing, response to steroid hormone, extracellular matrix organization, and endothelial cell migration (Figure 1D). During the active phase of Behçet’s syndrome, immunological and inflammatory factors cause endothelial cell dysfunction. Gene expression analysis identified two endothelial cell subtypes in aortic tissue: Endothelial cell1 and Endothelial cell2. In the BS group, 99.86% were Endothelial cell1, with only 0.14% being Endothelial cell2 (Figure 2A). Functionally, Endothelial cell1s were associated with antigen processing and presentation, while Endothelial cell2s were linked to small GTPase-mediated signal transduction and negative regulation of cell migration. KEGG pathway analysis showed significant enrichment of Epstein-Barr virus infection and antigen processing and presentation pathways in Endothelial cell1 of BS patients (Figure 2B). Mesenchymal cells are multipotent adult stem cells. In our study, the aortic mesenchymal cell count was 4,173 in the BS group and 1,433 in the control group. We identified three mesenchymal cell subgroups based on gene expression. The control group was mainly Mesenchymal cell3 (99.93%), while the BS group was predominantly Mesenchymal cell1 (65.54%) and Mesenchymal cell2 (34.41%) (Figure 2C). Biologic process analyses showed Mesenchymal cell1s were involved in muscle system processes, and Mesenchymal cell2s were associated with cell adhesion and vasculature development (Figure 2D).
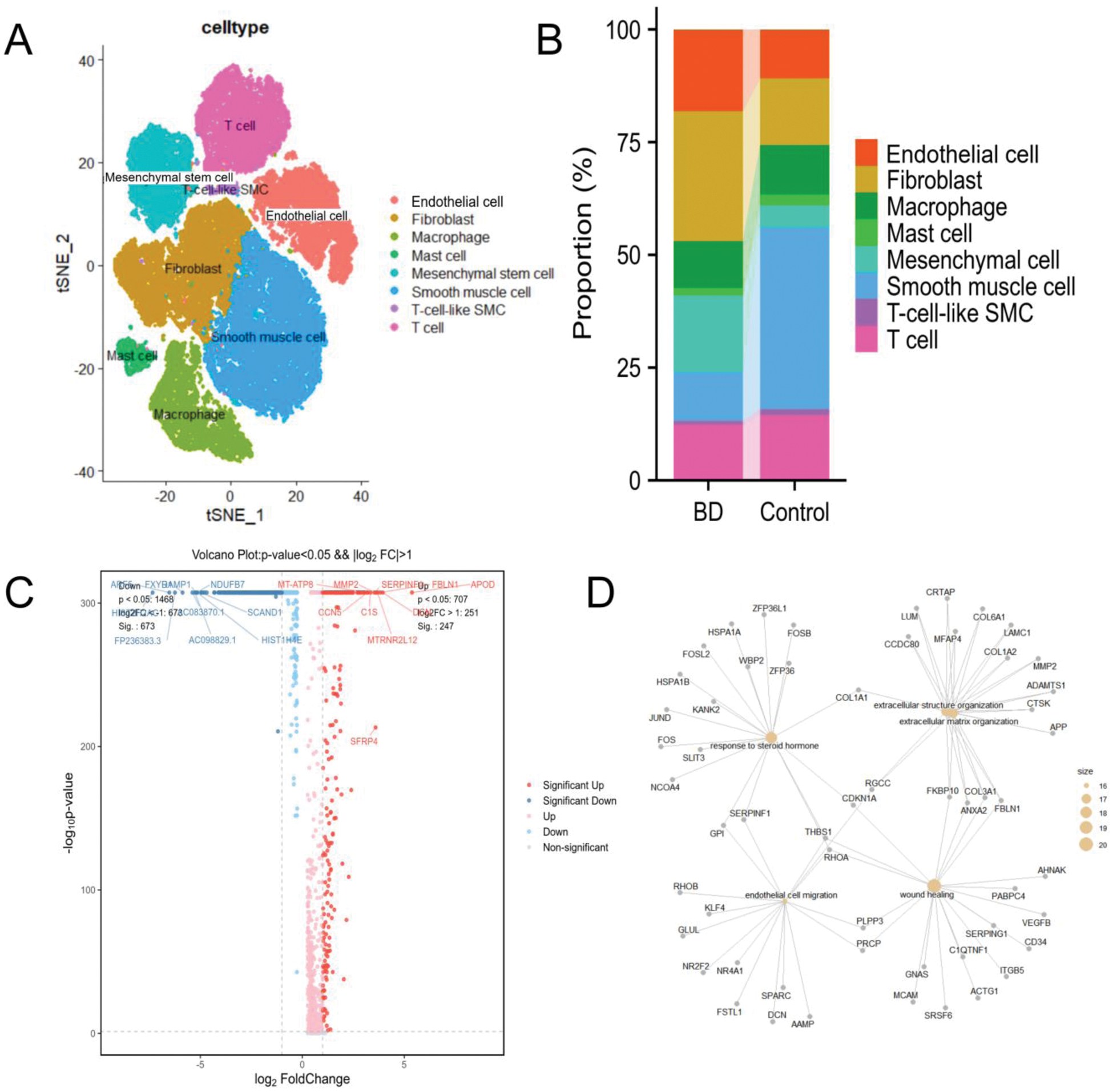
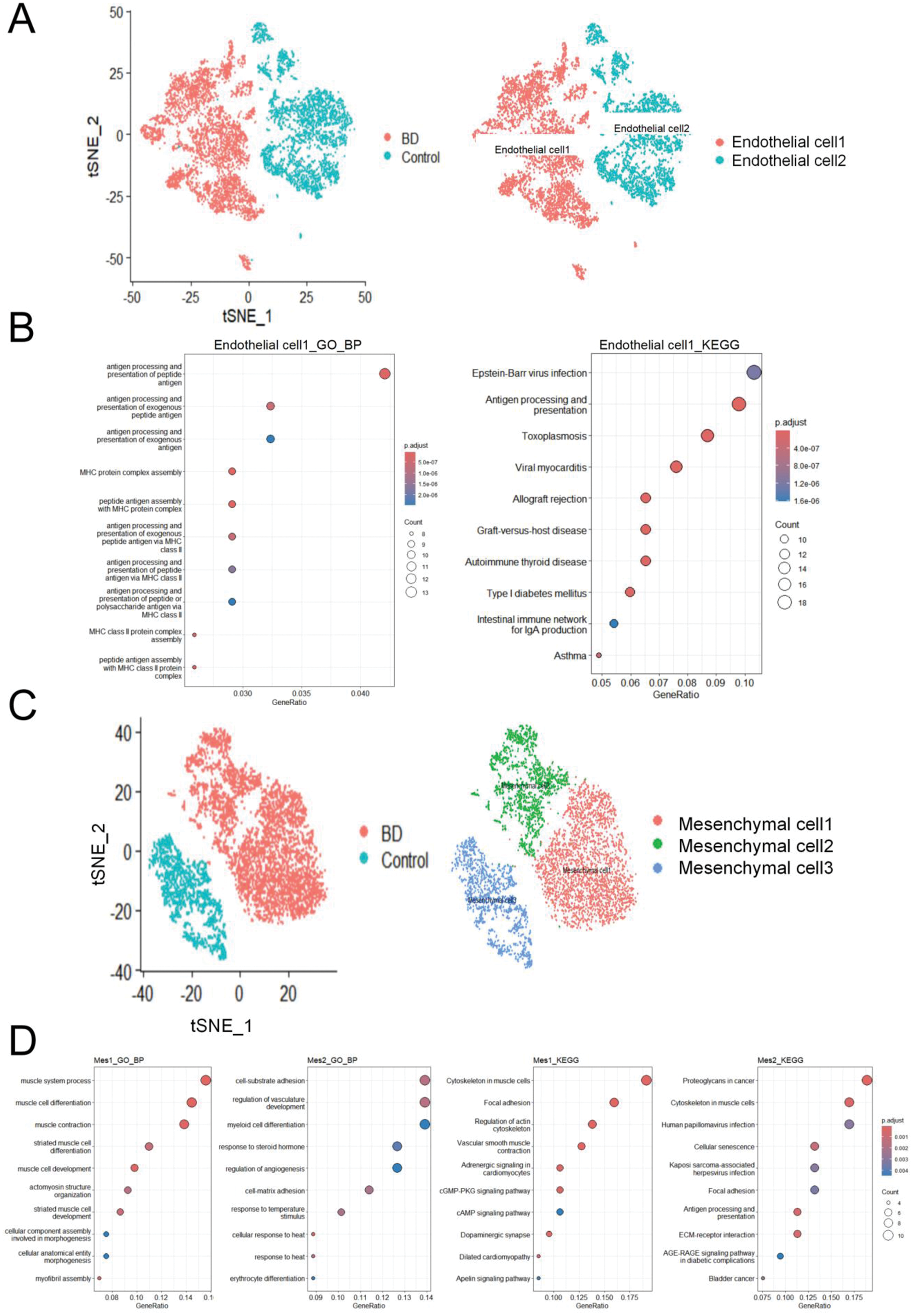
Conclusion: To our knowledge, this study is the first to comprehensively depict the single-cell transcriptional landscapes of aortic tissue in BS patients. Our single-cell sequencing analysis of aortic tissue from BS-related aortic aneurysm patients aligns with previous findings on non-BS aortic aneurysms, such as the significant reduction of smooth muscle cells in both cases. We also identified T-cell-like SMC, consistent with aortic aneurysm research. Prior studies have shown a close link between BS and endothelial cells, which are activated in BS patients, releasing inflammatory mediators and cytokines that cause vasculitis. Our study confirms that the number of endothelial cells in the aortic tissue of patients with BS is significantly higher than that in the control group, with endothelial cell1 being dominant and associated with antigen processing and presentation. The complex pathogenesis of BS involves genetics, immunity, environment, and infection. The increased processing and presentation of exogenous antigen by endothelial cells may be linked to infectious agents, as certain viral and bacterial antigens show significant homology with human proteins, potentially triggering cross-reactive immune responses in genetically predisposed individuals through molecular mimicry. Mesenchymal cell1, the predominant mesenchymal cell in BS patients’ aortic tissue, is mainly involved in muscle system processes, and we speculate it may compensate for the reduced smooth muscle cells. Further investigation is needed to explore the underlying mechanisms.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (