

Background: There are currently no effective pharmacological treatments for Sjögren’s disease (SjD). Our study aims to identify potential therapeutic targets for the condition using druggable genome-wide Mendelian randomization (MR).
Objectives: This study aims to identify druggable targets for SjD by integrating multi-omics datasets, including eQTL, mQTL, and pQTL analyses, with druggable genome data, Mendelian randomization (MR) analysis and Bayesian colocalization.
Methods: We integrated druggable genome data, blood cis-eQTL, cis-mQTL, cis-pQTL, and SjD GWAS summary data to identify druggable targets for SjD using the genome-wide MR approach. Bayesian colocalization was applied to validate shared causal genetic variants. Protein levels of prioritized genes were validated in clinical serum samples from SjD patients and controls using ELISA. Phenome-wide MR (Phe-MR) assessed side effects and alternative indications of identified targets.
Results: Fourteen druggable genes were identified, with eight (PLAT, SIRPB1, LAIR2, NEU1, SLC22A16, RAD52, PSPH, and CDH23) demonstrating consistent causal relationships with SjD. ELISA validation supported differential protein expression for these targets. Key findings include the protective role of NEU1 and PSPH in systemic immune regulation, the pathogenic impact of PLAT, SIRPB1, BRD2, and LAIR2 on inflammation, and the potential involvement of SLC22A16 and RAD52 in metabolic stress and immune activation. Actionable drugs, including Aminocaproic acid, Resveratrol, Levocarnitine, Imatinib, and Zinc chloride were identified as potential therapeutic candidates. No significant adverse effects were detected through Phe-MR analysis.
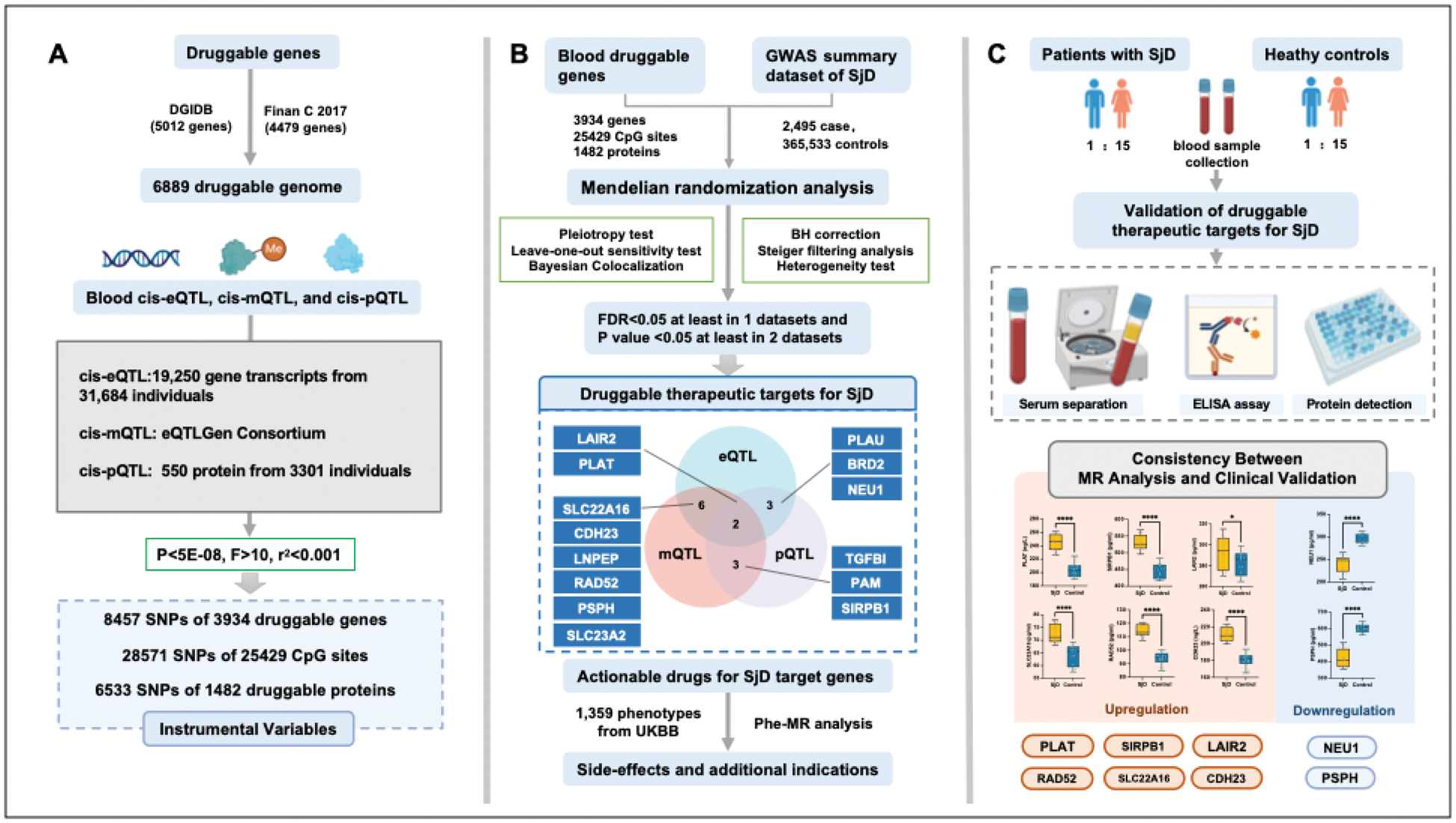
Conclusion: Our research indicated PLAT, SIRPB1, LAIR2, NEU1, SLC22A16, RAD52, PSPH, and CDH23 may serve as promising targets for SjD, while the effectiveness of Aminocaproic acid, Resveratrol, Levocarnitine, Imatinib, and Zinc chloride for SjD requires further validation.
REFERENCES: [1] Beydon M, McCoy S, Nguyen Y, Sumida T, Mariette X, Seror R. Epidemiology of Sjögren syndrome. Nat Rev Rheumatol. 2024;20(3):158-169. doi:10.1038/s41584-023-01057-6.
[2] Mariette X, Criswell LA. Primary Sjögren’s Syndrome. Solomon CG, ed. N Engl J Med. 2018;378(10):931-939. doi:10.1056/NEJMcp1702514.
[3] Seror R, Nocturne G, Mariette X. Current and future therapies for primary Sjögren syndrome. Nat Rev Rheumatol. 2021;17(8):475-486. doi:10.1038/s41584-021-00634-x.
[4] Baldini C, Fulvio G, La Rocca G, Ferro F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat Rev Rheumatol. 2024;20(8):473-491. doi:10.1038/s41584-024-01135-3.
[5] Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83. doi:10.1186/s13059-017-1215-1.
[6] Schadt EE, Björkegren JLM. NEW: network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4(115):115rv1. doi:10.1126/scitranslmed.3002132.
[7] Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Rev Genet. 2015;16(4):197-212. doi:10.1038/nrg3891.
[8] Fairfax BP, Humburg P, Makino S, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343(6175):1246949. doi:10.1126/science.1246949.
[9] Finan C, Gaulton A, Kruger FA, et al. The druggable genome and support for target identification and validation in drug development. Sci Transl Med. 2017;9(383):eaag1166. doi:10.1126/scitranslmed.aag1166.
[10] Santos R, Ursu O, Gaulton A, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16(1):19-34. doi:10.1038/nrd.2016.230.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (