

Background: inflammation and repair responses are intertwined processes in atherosclerosis. Although immune responses underlie vascular injury in rheumatoid arthritis (RA), thus accounting for cardiovascular risk excess, candidate mechanisms are to be identified. GPR55 is a cannabinoid receptor expressed in a variety of haematopoietic and stromal tissues. Recently, GPR55 signalling has been proposed to modulate atherosclerosis progression and vascular remodelling in knock-out animal models, but evidence from human studies is lacking.
Objectives: to evaluate the GPR55 expression in leukocyte populations in early RA patients and their potential role in atherosclerosis.
Methods: GPR55 expression on monocytes (CD14+ and CD14low subsets) and B-cells (CD19+) was quantified by flow cytometry in Peripheral Blood Mononuclear Cells (PBMCs) from 63 early, untreated RA patients (2010 EULAR/ACR classification criteria; 69% RF, 67% ACPA; DAS28: 5.32±1.14), 11 individuals with clinically-suspect arthralgia (CSA) (EULAR definition; 57% RF, 50% ACPA) and 36 matched healthy controls (HC). Public expression datasets were used for validation. Atherosclerosis occurrence was measured by Doppler-ultrasound. Serum levels of proinflammatory cytokines were evaluated by immunoassays. Serum proteins were evaluated through a pre-defined panel by using high-throughput targeted proteomics. Pathway analyses were performed under STRING with KEGG library. In vitro cultures were performed with PBMCs from healthy volunteers.
Results: GPR55 was detected on human monocytes and B-cells by flow cytometry. RA patients exhibited lower GPR55 expression (measured as mean fluorescence intensity) in monocyte subsets and B-cells compared to HC (Figure 1A). Equivalent results were obtained when the frequency of GPR55+ cells was computed (p=0.052, p=0.010 and p=0.010, respectively). No differences were observed between CSA individuals and HC (all p>0.050). Publicly available datasets using profiling array platforms confirmed a decreased expression of GPR55 in PBMCs (GSE17755 and GSE15573), but not in synovial tissue (GSE36700) from RA patients (Figure 1B). GPR55 expression was unrelated to disease activity, joint counts, symptom duration or traditional cardiovascular risk factors in RA patients (all p>0.050). However, smoking was associated to lower expression on B-cells (p=0.047), in a dose-dependent manner (cigarettes/day: r=-0.249, p=0.039) and restricted to patients carrying the Shared Epitope (p<0.050, and r=-0.375, p=0.027; respectively). Atherosclerosis occurrence or cIMT were not associated with GPR55 expression on B-cells (p=0.174, and r=0.027, p=0.841, respectively), nor in monocyte subsets (all p>0.050). GPR55 expression on B-cells was correlated with serum levels of proinflammatory cytokines (IL6: r=0.236, p=0.062; IL-18: r=0.410, p<0.001, and IL-8: r=0.317, p=0.011) in RA. Furthermore, GPR55 expression on B-cells was associated with several proteins related to vascular remodelling (PGF, CXCL1, IL-18, SERPINA12, MMP7, THBS2 and MMP12) and B-cell activation/differentiation (SLAMF7, IL-6, TNFRSF13B or VSIG2) (Figure 1C). These proteins informed signatures (protein-protein interaction: p=5.99·10 -11 ) related to humoral adaptive responses, cytokine production and regulation of defence response. However, these associations were atherosclerosis-dependent, and were only restricted to patients with atherosclerosis (n=35), where specific proteomic pathways involved in the “positive regulation of cytokine production” and “cellular response to lipopolysaccharide (LPS)” predominated. In vitro cultures demonstrated that LPS treatment (from 10 to 5000 ng/ml) decreased GPR55 expression on B-cells, in a dose-dependent manner (r=-0.832, p for trend<0.001) and even at lower doses. This effect overlapped with increasing CD86 expression (r=0.430, p for trend=0.036) (Figure 1D). No effects on B-cell numbers or cell viability were registered across LPS doses (all p>0.050). This effect was early (24h) and sustained along longer timepoints (48 and 72h).
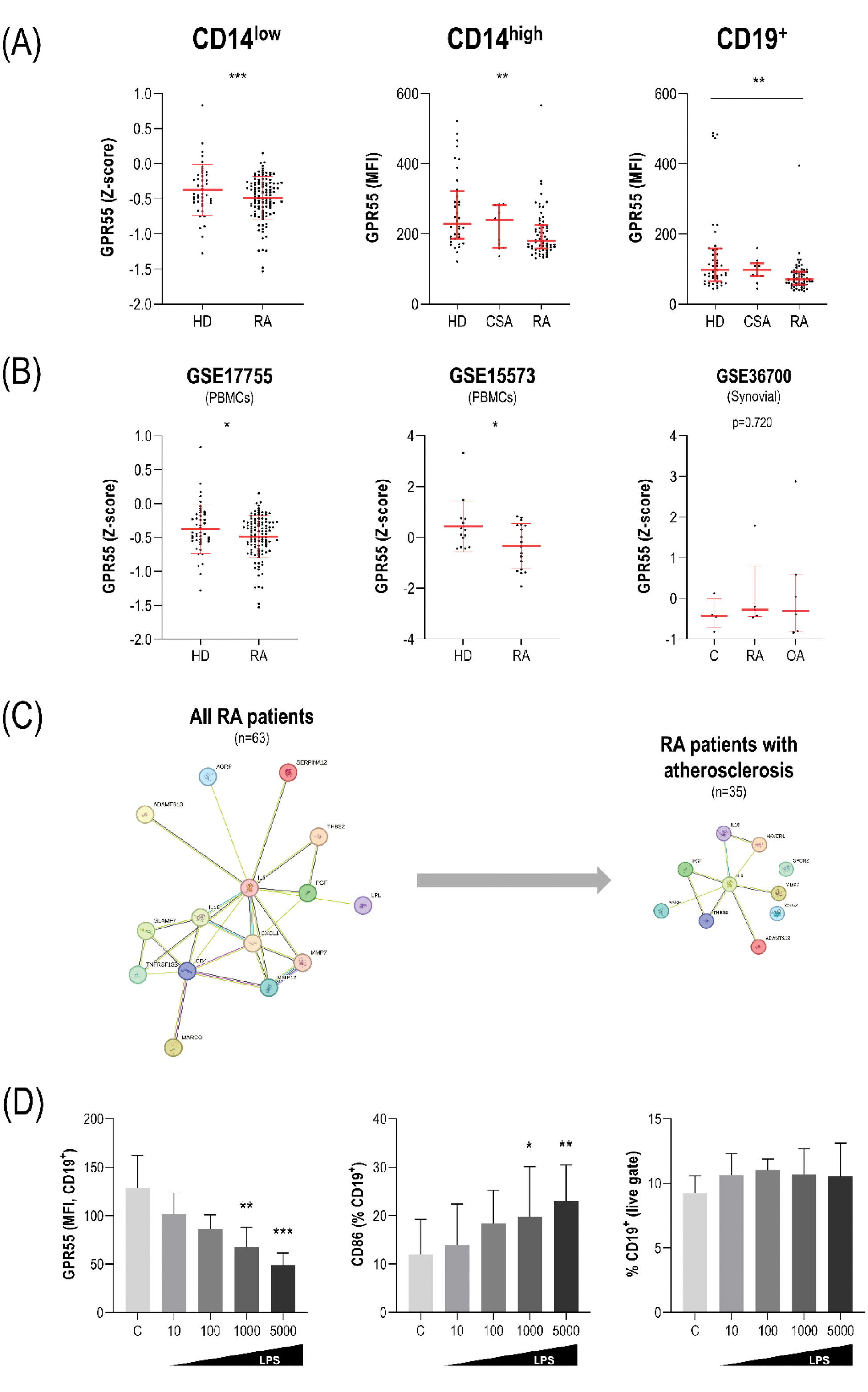
Conclusion: Reduced GPR55 expression hallmarked monocyte and B-cell subsets in RA patients at onset, whereas no differences were found in the preclinical stage. GPR55 expression was linked to B-cell activation-related pathways, especially in patients with atherosclerosis, and presumably via T-cell independent mechanisms. GPR55 may be a novel hub to understand the crosstalk between immune responses and maladaptive remodelling in atherosclerosis. This is the first study assessing GPR55 expression in patients with rheumatic and musculoskeletal conditions.
REFERENCES: NIL.
Acknowledgements: ISCIII (PI reference PI21/00054; and PFIS reference FI22/00148),
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (