

Background: Extracellular vesicles (EVs) are lipid bilayer-enclosed nanoparticles secreted by cells, present in various body fluids. They play a central role in intercellular communication and hold potential as future biomarkers for numerous diseases, including rheumatoid arthritis (RA). Increasing evidence highlights the involvement of EVs in the pathogenesis of RA [1]; however, their prognostic significance remains unclear. Our research group was the first to describe and publish direct experimental evidence of the formation of a protein corona around EVs in human plasma [2, 3]. In this study, we investigated the presence of citrullinated proteins within the EV-associated protein corona.
Objectives: The objective of this study is to compare the protein corona of extracellular vesicles (EVs) in samples from RA patients, healthy controls, and patients with osteoarthritis (OA). This analysis aimed to investigate the relationship between EV corona citrullination and the diagnosis, prognosis, disease activity, and pathogenesis of RA.
Methods: In this study, we established five groups: healthy donors, patients with OA, and three distinct RA patient populations. The RA groups were categorized as follows:
PreRA individuals who were anti-citrullinated protein antibody (ACPA) positive with arthralgia.
Patients with at least moderate disease activity (DAS28 ≥ 3.2; disease duration >1 year).
Patients in sustained remission (DAS28 < 2.6; remission duration >1 year).
The blood samples were collected in tubes containing trisodium citrate, citric acid, and dextrose, and platelet-free plasma was prepared. Extracellular vesicles (EVs) were isolated using differential ultracentrifugation (dUC), density gradient ultracentrifugation (DGUC), and size exclusion chromatography (SEC). Sample validation was performed according to the MISEV 2023 recommendations. Samples were divided into two equal aliquots: one was left untreated, while the other was treated with protein corona stripping buffer. The protein composition of the samples was analyzed using mass spectrometry (MS).
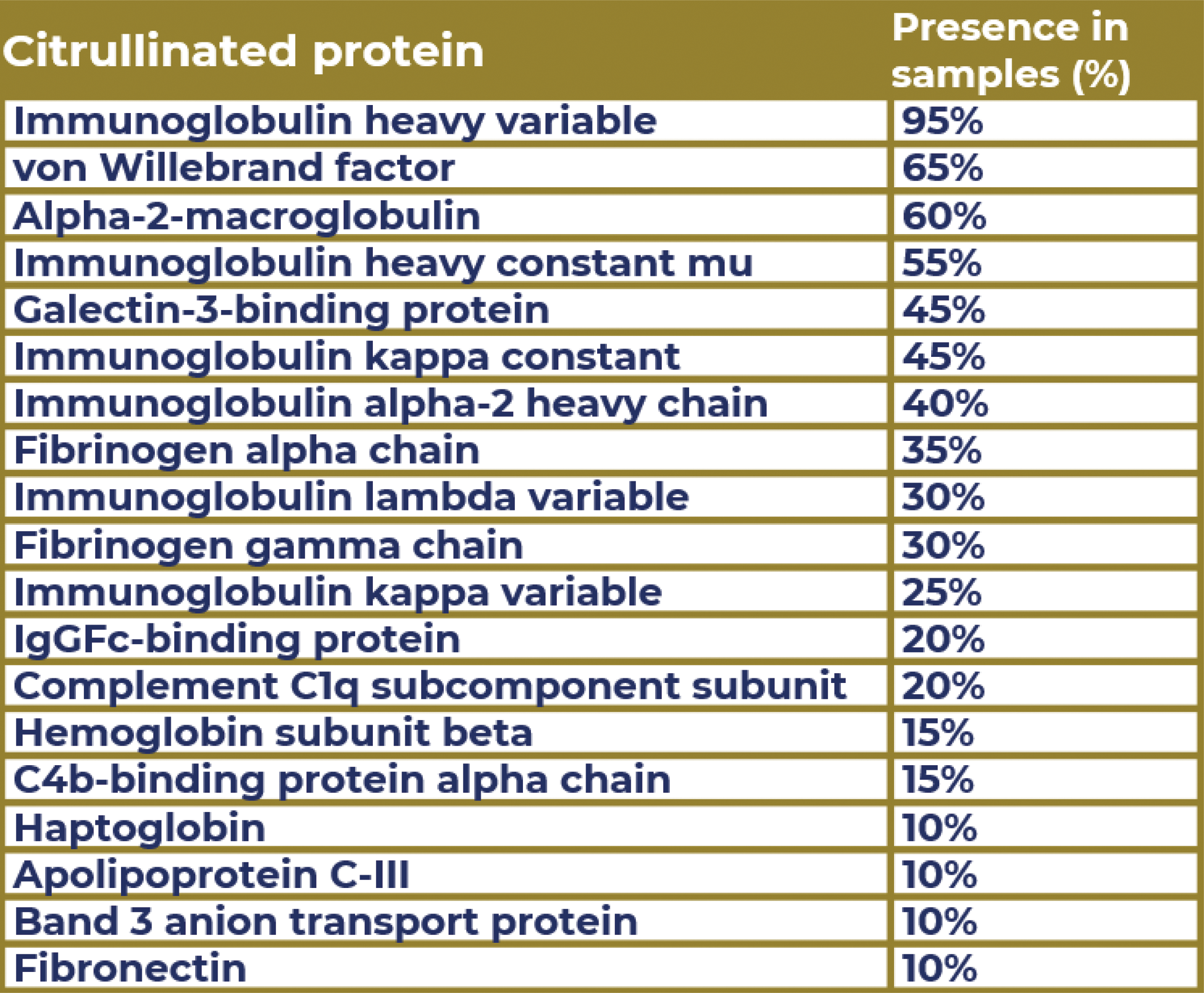
Results: The presence of extracellular vesicles was confirmed using transmission electron microscopy (Figure 1) and flow cytometry. Multiple citrullinated proteins were identified in the EV samples (n=20) from RA donors using MS, including apolipoproteins C and E, complement components C1q and C4, galectin-3 binding protein, haptoglobin, actin, and aquaporin-1 (Table 1). Additionally, in RA patients, citrullinated proteins form part of the protein corona, such as von Willebrand factor, fibrinogen, fibronectin, and various immunoglobulins. To our knowledge, we are the first to report the presence of citrullinated peptides within the protein corona of EVs in RA patients. As of the preparation of this abstract, mass spectrometric analysis is ongoing for samples (n=366) from all donors (n=61). These results will include all group-specific proteins identified, particularly focusing on the citrullinated corona proteins.

Table 1.
Conclusion: Citrullinated proteins are present in the protein corona of EVs in RA patients. Following further investigations, these proteins may serve as potential diagnostic or prognostic biomarkers.
REFERENCES: [1] Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014 Jun;10(6):356-64.
[2] Tóth, E. Á., Turiák, L., Visnovitz, T., Cserép, C., Mázló, A., Sódar, B. W.,. & Buzás, E. I. (2021). Formation of a protein corona on the surface of extracellular vesicles in blood plasma. Journal of extracellular vesicles, 10(11), e12140.
[3] Buzas EI. (2022) Opportunities and challenges in studying the extracellular vesicle corona. Nat Cell Biol.24:1322-1325.
Acknowledgements: This research was funded by the OTKA K131479, NVKP_16-1-2016-0004, VEKOP-2.3.2-162016-00002, VEKOP-2.3.3-15-2017-00016, the Therapeutic Thematic Programme TKP2021-EGA-23, RRF-2.3.121-2022-00003 (National Cardiovascular Laboratory Program) and 2019-2.1.7-ERA-NET-2021-00015. The project has received funding from the EU’s Horizon 2020 Research and Innovation Programme under grant agreement No. 739593 and the NKFIH Advanced grant 150767.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (