

Background: A pathogenic role for type I interferons (IFN) has been hypothesized across multiple autoimmune indications, including rheumatoid arthritis (RA). Evidence in the literature shows that an elevated type I IFN gene signature (IFNGS) is present in the peripheral blood and synovial fluid of approximately 10-30% of RA patients [1, 2]. A high IFNGS may be associated with the development of disease in pre-symptomatic patients [3], and has been shown to correlate with DAS28 in early RA patients [4]. Additionally, increased IFN activity may negatively impact response to treatment in both early and established RA patient populations [5, 6]. However, small cohort sizes and variability in the IFN gene signatures used in these studies make it challenging to understand the role of IFN in RA.
Objectives: To evaluate the size of IFN-high RA sub-populations in early and established RA patients and to understand how treatment may impact their peripheral blood IFNGS score.
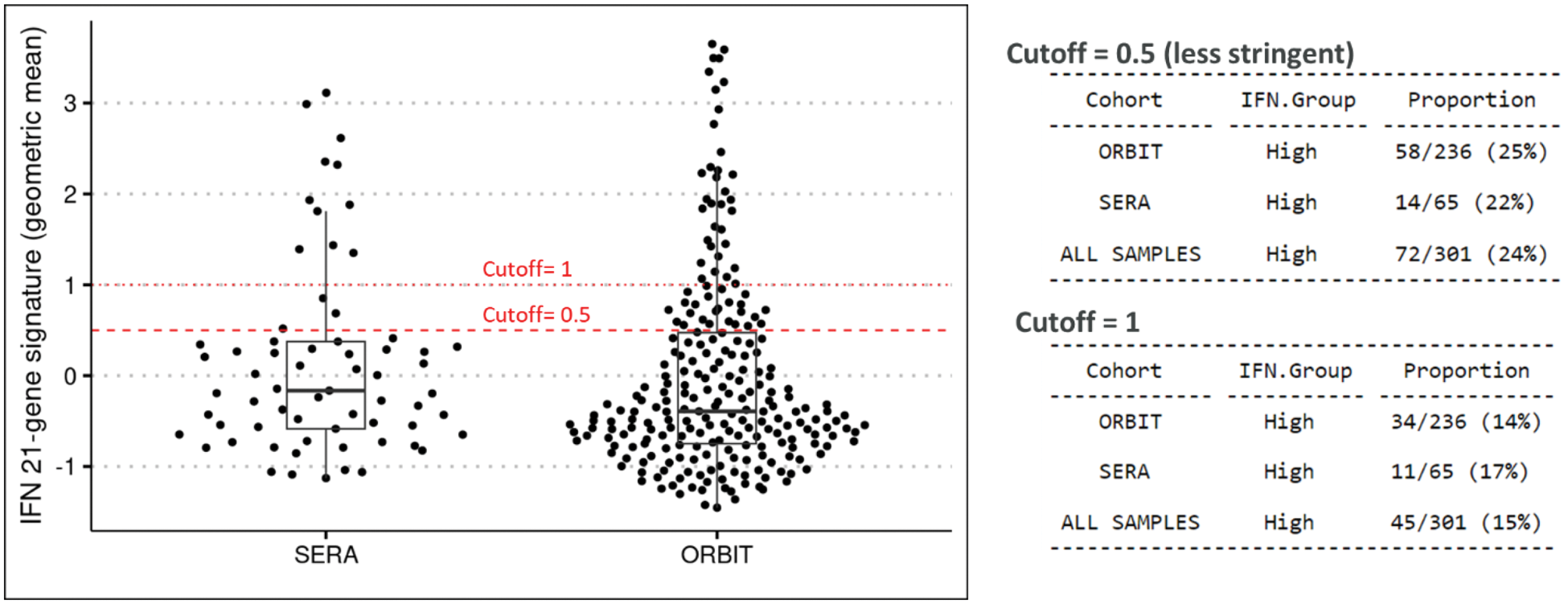
Methods: Patient cohorts established by the University of Glasgow included 1) early, treatment-naive RA patients who were initiated on csDMARDs (SERA cohort [7]); all patients received methotrexate; and 2) established RA patients who had failed methotrexate therapy and were randomized to receive either anti-TNF (adalimumab or etanercept) or rituximab therapy (ORBIT cohort [8]). For SERA, treatment response was determined based on % change in CDAI scores with good responders achieving ≥85% reduction and non-responders having <50% reduction. For ORBIT, treatment response was determined using the EULAR criteria, from which good responders were defined by those achieving either good response (same criteria as above) or remission post-treatment. Partial responders were excluded from analysis. RNA sequencing was performed on peripheral whole blood samples collected at baseline and month 6 (SERA) or month 3 (ORBIT) post-treatment. The geometric mean of 21 type I IFN-induced genes (IFN21) [9] was calculated for each patient and values were scaled within each cohort. IFN-high and IFN-low subpopulations were identified in both cohorts using a cut-off of 0.5 established from a prior study in SLE [10]. Statistical testing between populations were computed using a paired t-test or a chi-squared test where appropriate.
Results: Elevated IFN21 gene signature score was present in 15 to 25% of RA patients at baseline in the SERA and ORBIT cohorts, depending on the cut-off value applied to the data, with no difference in prevalence observed between early and established RA patients (Figure 1). Baseline type I IFN gene signature score did not correlate with disease activity, nor did it distinguish between good and non-responders in either cohort, although there was a trend towards lower response rate to anti-TNF or rituximab treatment in the IFN-high ORBIT subgroups. Early RA patients in the IFN-high subgroup who were good-responders had a non-significant decrease of IFN21 gene signature score after six months of treatment. Conversely, the IFN21 gene signature score tended to increase in established RA patients in the IFN-low subgroup after three months of anti-TNF treatment, although the majority of these patients remained IFN-low. Limitations to the study include small sample size; additional cohorts will be needed to confirm these results.
Conclusion: The expression of type I IFN stimulated genes is elevated in a subgroup of RA patients, both in early pre-treatment disease and in established RA patients. Additional molecular and cellular phenotyping is needed to further characterize these patients and to better establish the role that IFN plays in the pathogenesis of RA.
REFERENCES: [1] Ann Rheum Dis 2011;70:2029-2036.
[2] Ann Rheum Dis 2007;66:1008–1014.
[3] Ann Rheum Dis 2013;72:776–780.
[4] JACI. 2018 Jan;141(1):445-448.
[5] Arthritis Res Ther. 2016 Dec 12;18(1):290.
[6] Arthritis ResTher. 2012, 14: R95.
[7] BMC Musculoskeletal Disorders, 17(1), 461.
[8] Lancet. 2016 Jul 16;388(10041):239-47.
[9] Hum Genomics Proteomics 2009; 2009: 374312.
[10] Ann Rheum Dis 2024; 0:1–10.
Prevalence of patients with elevated IFN signature at baseline in early (SERA) and established (ORBIT) RA.
Acknowledgements: NIL.
Disclosure of Interests: Eszter Csomor AstraZeneca, AstraZeneca, Arun Jayaraman AstraZeneca, AstraZeneca, Lynn Stewart: None declared , Fraser Morton: None declared , Caron Paterson salary was paid by a Pfizer grant (for the SERA study) from April 2011 to June 2015, Greet DeBaets AstraZeneca, AstraZeneca, Daniel Muthas AstraZeneca, AstraZeneca, Jessica Neisen AstraZeneca, AstraZeneca, Mia Collins AstraZeneca, AstraZeneca, Rachel Sparks AstraZeneca, AstraZeneca, Philip Z. Brohawn AstraZeneca, AstraZeneca, Chris Chamberlain AstraZeneca, UCB, Roche, AstraZeneca. UCB, Roche, Aurelie Najm AstraZeneca, Adam Platt AstraZeneca, AstraZeneca, Iain B. McInnes Abbvie, UCB, Janssen, Novartis, Abbvie, Astrazeneca, Absci, Amgen, BMS, Boerhinger, Causeway Therapeutics, Cabaletta, Compugen, Dextera, Eli Lilly, Gilead, Janssen, Montai, Moonlake, Novartis, Pfizer, Roche, Recludix, Sanofi, UCB, Lilly, Astra Zeneca, Dextera, Novartis, GSK, Carl S Goodyear AstraZeneca.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (