

Background: Osteoarthritis (OA) is a debilitating joint disease characterized by synovial inflammation, cartilage damage, subchondral bone change, and osteophyte formation. Emerging evidence identifies synovial inflammation as an early pathological change.
Objectives: This study investigates the regulatory role of NOD2 palmitoylation in macrophage activation and its impact on OA progression, focusing on the selective autophagy receptor p62.
Methods: Quantitative PCR (qPCR) and Western Blot (WB) analyses were conducted to evaluate NOD2 expression at mRNA and protein levels under high concentrations of 2BP, a palmitoylation inhibitor. Chloroquine (CQ) and MG132 were used to delineate the roles of the autophagy-lysosome and ubiquitin- proteasome pathways in NOD2 degradation. Immunoprecipitation (IP) assays identified interactions between NOD2 and selective autophagy receptors (SARs), including p62. Immunohistochemistry (IHC) was performed on synovial tissues from OA patients and healthy controls to assess p62 expression. Lentiviral vectors were used to overexpress p62 in macrophages in vitro, and ELISA and confocal microscopy were employed to evaluate pro-inflammatory cytokine levels. A collagenase-induced osteoarthritis (CIOA) model was established in 8-week-old C57BL/6J mice to investigate the effects of p62 overexpression on OA progression.
Results: High dose 2BP significantly reduced NOD2 protein levels without affecting its mRNA expression, indicating post-translational regulation. CQ, but not MG132, reversed 2BP-induced NOD2 degradation, implicating the autophagy-lysosome pathway. Among SARs, p62 exhibited the strongest interaction with NOD2, and this interaction negatively correlated with NOD2 palmitoylation levels. IHC analysis revealed elevated p62 expression in OA synovial tissues. Overexpression of p62 in macrophages significantly increased TNF-α secretion, while ZDHHC5 overexpression reduced its elevation. In the CIOA mouse model, intra-articular injection of lentivirus targeting p62 delayed the progression of osteoarthritis.
Conclusion: Our findings suggest that p62 mediates NOD2 degradation via the autophagy-lysosome pathway, enhancing macrophage activation and promoting OA progression. This highlights p62 as a potential therapeutic target for OA, warranting further translational research.
REFERENCES: NIL.
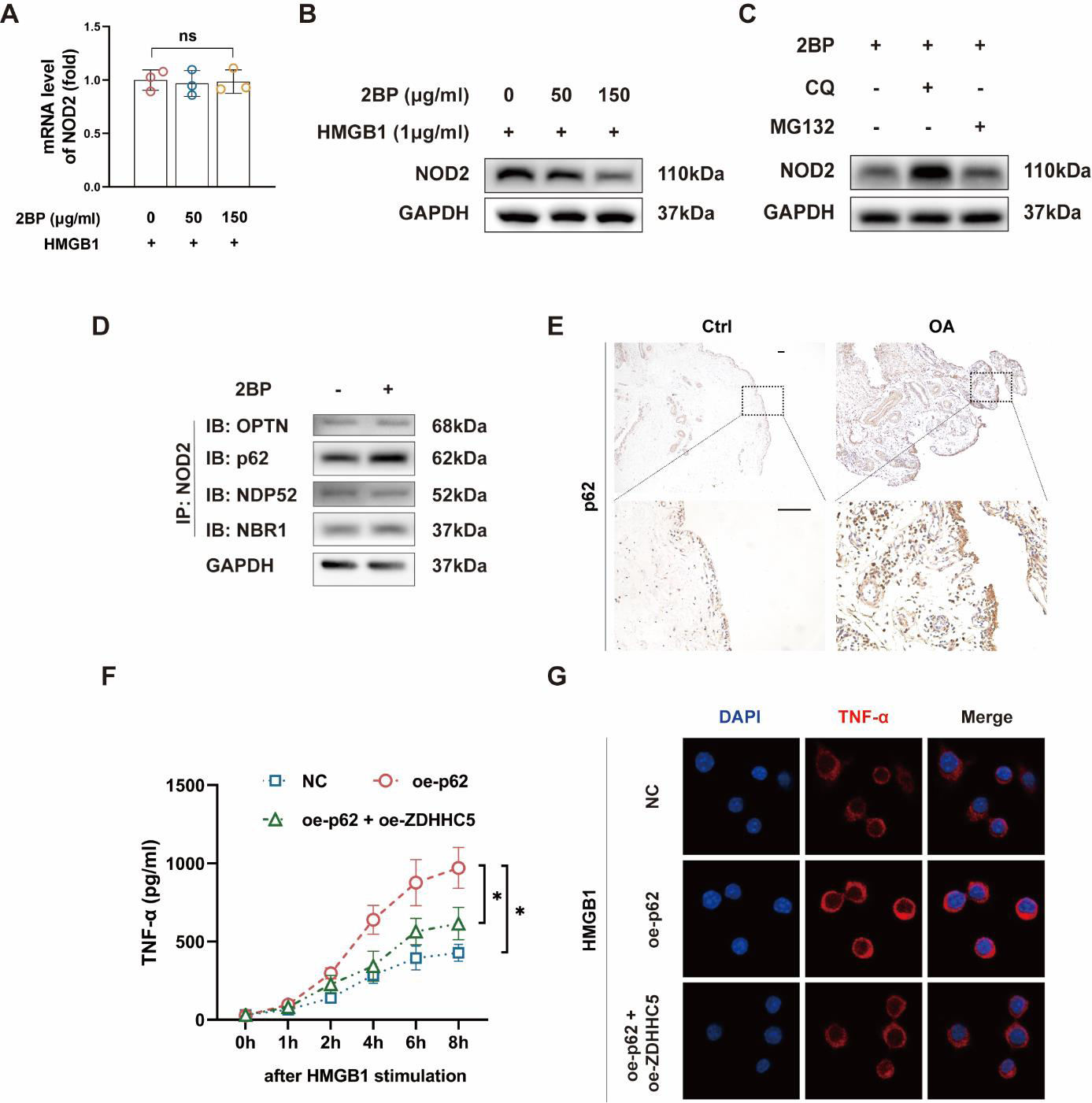
Selective autophagy receptor p62 promotes macrophage activation.
A The palmitoylation inhibitor 2BP does not affect the mRNA expression of NOD2.
B High concentrations of 2BP (150 μg/ml) significantly reduce the protein levels of NOD2.
C The 2BP-mediated downregulation of NOD2 can be rescued by the autophagy-lysosome pathway inhibitor CQ, but not by the ubiquitin-proteasome pathway inhibitor M132.
D NOD2 interacts with the selective autophagy receptor p62.
E The expression of the selective autophagy receptor p62 in the synovium of osteoarthritis patients is significantly higher than in healthy controls.
F, G Overexpression of p62 significantly upregulates the expression and release of inflammatory cytokine TNF-α in macrophages in vitro, which effect is rescued by overexpression of ZDHHC5.
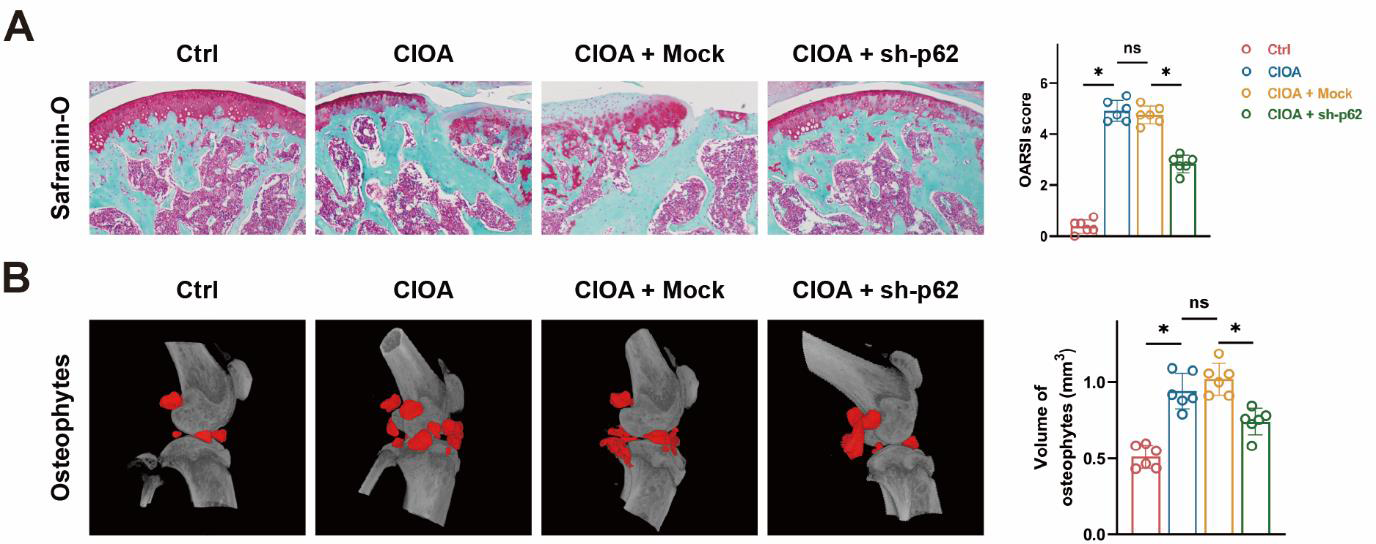
In vivo inhibition of p62 alleviates osteoarthritis progression in mice. A Inhibition of p62 in mice mitigates cartilage damage and improves the OARSI score. B In vivo p62 inhibition in mice significantly reduces osteophyte formation.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (