

Background: Gastrointestinal tract (GIT) involvement is highly prevalent among patients with systemic sclerosis (SSc) and is associated with high morbidity and mortality. Understanding how the aberrant molecular mechanisms differ across early and late-stage disease, subtypes, and autoantibody profiles is limited. Filling these knowledge gaps is critical to identifying and implementing novel treatment options.
Objectives: Our study aimed to assess molecular alterations in the duodenal tissue of SSc patients across different disease stages, subtypes, and autoantibody profiles compared to control tissue.
Methods: We analyzed gastroduodenal biopsies from SSc patients (n=30) enrolled in the ReSScue trial. Patients presented moderate to severe lower gastrointestinal tract involvement. Donors with non-celiac gluten sensitivity were included as controls (n=10). Tissue morphology and structure were assessed using Hematoxylin and Eosin (H&E) staining. Inflammatory markers included anti-CD45 for leukocytes, anti-CD68 for macrophages, anti-CD3 for T-cells, and anti-CD20 for B-cells. Collagen deposition was evaluated by sirius red staining and further characterized with immunohistochemistry (IHC) using anti-collagen I antibodies. Lymphatic vessels and blood vessels were identified using anti-podoplanin and anti-CD34 antibodies, respectively. The proportion of tissue stained with sirius red and collagen I was quantified using Orbit Image Analysis, while QuPath software was employed to determine the percentage of positively stained cells in IHC analyses. The expression of fibrotic, inflammatory and vascular markers was correlated to clinical parameters. Statistical analyses were performed with GraphPad Prism 10, using the Mann-Whitney test.
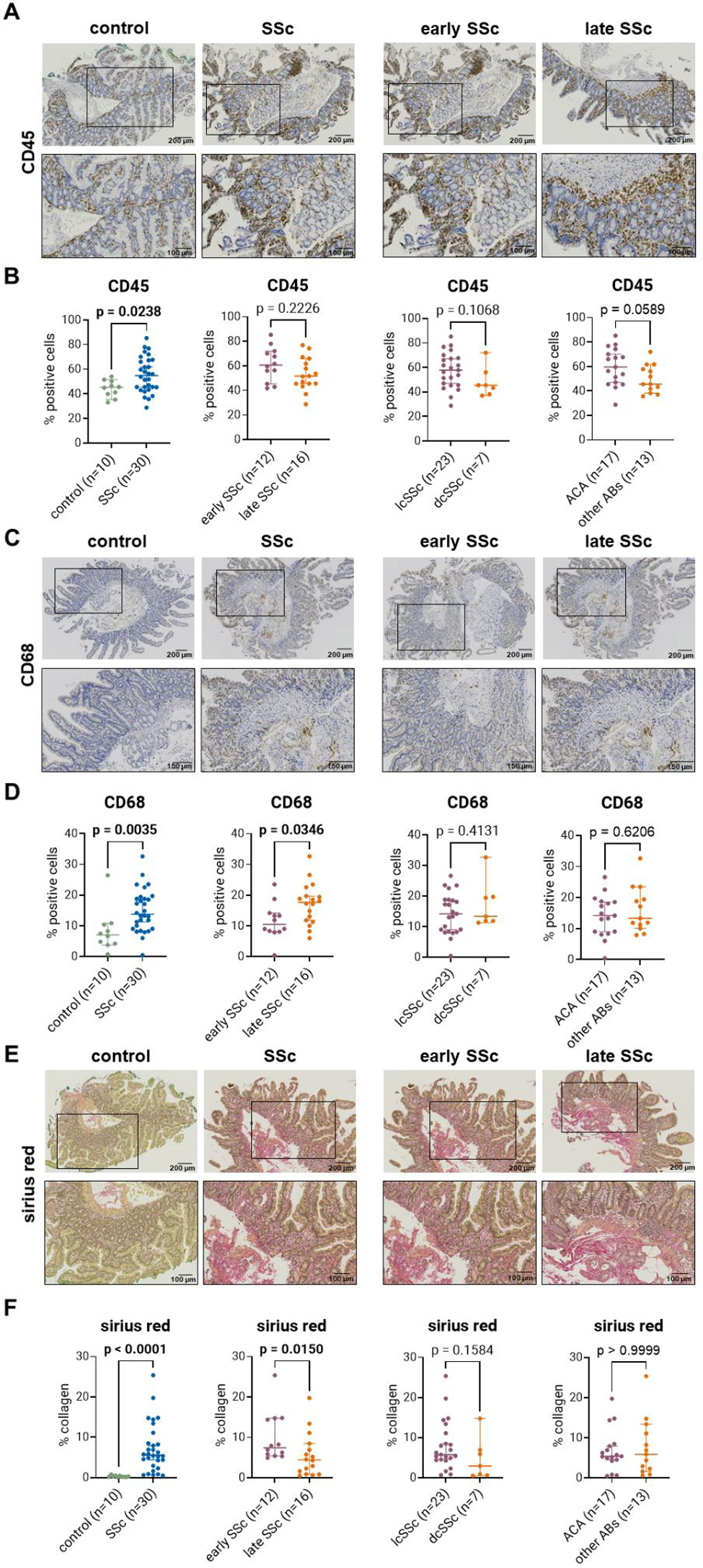
Results: When comparing SSc with controls, we found increased inflammation in SSc patients with an elevated number of leukocytes (54.9% versus 45.3%; p < 0.024; Figure 1A+B) and CD68-positive macrophages (13.7% versus 7.1%; p = 0.0035; Figure 1C+D). T-cells were significantly reduced in SSc compared to controls (24.5% versus 34.6%; p = 0.0006), but we did not observe significant differences in CD20-positive B-cells. We identified increased collagen deposition stained by sirius red (5.6% versus 0.25%; p < 0.0001; Figure 1E+F) and significantly increased levels of collagen I (9.8% versus 7.0%; p = 0.026). Lastly, there were no significant differences for the vascular markers podoplanin and CD34. We then compared early SSc patients (n=12; mean disease duration 2.2 ± 1.2 years) and patients with late disease (n=16; mean disease duration 19.4 ± 5.8 years). These two groups did not differ in other clinical parameters (Table 1). Interestingly, early SSc patients showed significantly less CD68-positive macrophages as compared to late-stage patients (10.5% versus 17.7%; p = 0.035; Figure 1C+D). No significant differences were detected between early and late SSc patients for all other inflammatory markers. Both early and late SSc patients showed increased collagen deposition as detected by sirius red staining (7.4% versus 4.4%; p = 0.015; Figure 1E+F) and collagen I (11.2% versus 8.4%; p = 0.013). No differences were found for vascular markers between the groups. Next, we compared limited cutaneous SSc (lcSSc) patients (n=23) to diffuse cutaneous SSc (dcSSc) patients (n=7) and patients with anti-centromere antibody (ACA) (n=17) to patients with any other type of seropositivity (n=13; Table 1). We did not observe any significant differences in respect to any of the markers for inflammation, fibrosis, or vascular changes. When comparing patients taking immunosuppressants to those not taking them, we could observe a trend of decreased CD45-positive leukocytes in the immunosuppressant group (57.2% versus 48.5%; p = 0.17). We could not find any correlations between inflammation, collagen deposition or vasculopathy and lower GIT symptoms distention/bloating and diarrhea assessed by the UCLA GIT 2.0 score.
Table 1. Characteristics and clinical data for SSc patients with early and late disease, lcSSc and dcSSc, as well as ACA and other autoantibodies. Early SSc patients have a disease duration < 5 years and late SSc patients have a disease duration > 10 years. mRSS = modified Rodnan skin score, ILD = Interstitial lung disease, AB = antibody.
Histological and IHC analysis of fibrotic and inflammatory markers (sirius red, CD45 and CD68) and representative images of duodenal tissue of SSc patients with early and late-stage disease, lcSSc and dcSSc as well as ACA and other autoantibodies. Statistical analyses were performed with GraphPad Prism 10, using the Mann-Whitney test.
Conclusion: Our data demonstrate the presence of fibrosis and inflammation in duodenal biopsies of SSc patients both in patients with early and late-stage disease and across all SSc subtypes. The presence of fibrosis early in the disease and sustained inflammation over the disease course could inform gut-targeted treatment decisions. Transcriptomic and proteomic analysis of SSc GIT tissue will provide more insight into the molecular alterations of GIT involvement in SSc to identify novel treatment targets.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Laura Much: None declared, Elena Pachera: None declared, Håvard Fretheim: None declared, Andrea Laimbacher: None declared, Knut EA Lundin: None declared, Lars Aabakken: None declared, Lumeng Li: None declared, Astrid Hofman: None declared, Pietro Bearzi: None declared, Michael Scharl: None declared, Øyvind Molberg: None declared, Oliver Distler OD has/had consultancy relationships with and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, Co-founder of CITUS AG, OD has/had consultancy relationships with and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Acepodia Biotech, Aera, Alcimed, Altavant, Amgen, AnaMar, Anaveon AG, Argenx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Calluna (Arxx), Cantargia AB, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, MSD Merck, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta Inc., Novartis, Orion, Pilan, Prometheus, Quell, Redxpharma, Roivant, EMD Serono, Topadur and UCB, Research Grants: BI, Kymera, Mitsubishi Tanabe, UCB, Anna-Maria Hoffmann-Vold Boehringer Ingelheim, Janssen, Medscape, Merck Sharp & Dohme, Novartis and Roche, AbbVie, ARXX, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Janssen, Medscape, Merck Sharp & Dohme, Pliant Therapeutics, Roche and Werfen, Boehringer Ingelheim, Janssen.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (