

Background: Catastrophic antiphospholipid syndrome (CAPS) is a rare but lethal condition characterized by micro and macrovascular occlusions in a short period, which affects approximately 1% of patients with antiphospholipid syndrome (APS). In Mexico, a prevalence of 2 cases per 10,000,000 inhabitants with a mortality rate of 68% has been estimated. However, it is considered that this entity is underreported.
Objectives: To describe the clinical characteristics and outcomes of patients with CAPS in a tertiary-care center.
Methods: Retrospective study from 2018-2023. Patients diagnosed with CAPS treated at a tertiary-care center were included. Demographic data, clinical and laboratory characteristics, and outcomes were collected from the electronic medical records. The Sydney 2006 and preliminary classification criteria were used for the diagnosis of APS and CAPS, respectively. Descriptive statistics were used, Stata V14 was used for statistical analysis.
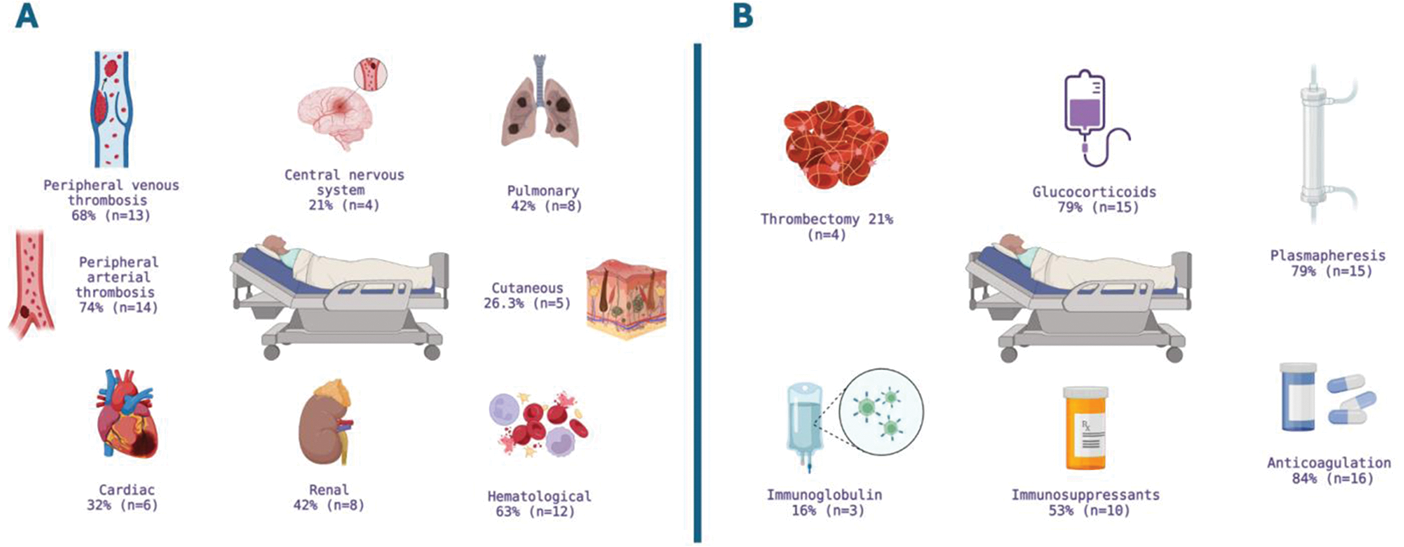
Results: Nineteen patients with CAPS diagnosis were identified during the analyzed period. Seventy-four percent (n=14) were women, with a mean age of 38.4 years (SD=12.7). Sixty-eight percent (n=13) had secondary APS, with systemic lupus erythematosus being the most frequent rheumatological disease in 84.6% (n=11) of these cases. Eighty-four percent (n=16) of patients had presented thrombotic events prior to the CAPS event, 37% (n=7) had hematological manifestations, 39% (n=7) had non-criteria manifestations, and 29% (n=4) had obstetric manifestations. Forty-two percent (n=8) had comorbidities, with obesity being the most common in 32% (n=6). The Figure 1 (panel A) describes the frequency of organ involvement. Thirty-two percent (n=6) of cases were classified as definitive and 68% (n=13) as probable CAPS, respectively. The median time from APS diagnosis to CAPS presentation was one month (IQR=0-36 months). All patients developed multiorgan failure within the first seven days, and biopsies were performed in 33% (n=6) of cases, with the skin being the most common site (83.3%, n=5) and the most frequent histopathological report being occlusive thrombotic vasculopathy. A trigger for CAPS was identified in 63% (n=12) of cases, with infection being the main trigger (58%, n=11). The main laboratory abnormalities and treatment instituted are summarized in Figure 1 (panel B). Seven patients (37%) underwent amputation as part of the treatment. Twelve patients (63%) required ICU admission, with a median length of hospital stay of 26 days (IQR=16-47). ICU stay was two days (IQR=0-10), and there was a mortality rate of 42% (n=8).
Conclusion: To our knowledge, this is the largest case series of CAPS patients reported in Mexico. The 42% mortality rate found was almost the same as those reported in world literature (37%) and lower than the previous rate reported in Mexico (68%). This could be explained by earlier identification and treatment of this entity. Reporting these cases contributes to knowledge generation, especially in rare entities like CAPS.
Disease characteristics and acute event characteristics of CAPS Patients. (n=19)
| Total population
| CAPS Registry
|
|
|---|---|---|
| Sociodemographic Characteristics | ||
| Female | 14 (74) | 343 (69%) |
| Age, year, mean (SD) | 38.4 (12.7) | 38 |
| Smoking | 8 (42) | |
| Comorbidities | 8 (42) | |
| Obesity a | 6 (32) | |
| Systemic arterial hypertension | 3 (16) | |
| Coronary artery disease | 2 (10.5) | |
| Neoplasm | 2 (10.5) | |
| Diabetes Mellitus Type 2 | 1 (5) | |
| Disease-Specific Characteristics | ||
| Primary APS | 6 (32) | (60) |
| Secondary APS
| 13 (68)
| (40)
|
| APS manifestations before CAPS | ||
| Thrombotic manifestations | 16 (84) | |
| Hematological manifestations | 7 (37) | |
| Obstetric manifestations | 4 (29) | |
| Non-criteria manifestations | 7 (37) | |
| Immunosuppressive treatment | 15 (79) | |
| Glucocorticoid use | 14 (74) | |
| Prednisone dose, mg, median (IQR) | 17.5 (5-50) | |
| Total anticoagulation | 7 (37) | |
| Antiplatelet therapy | 3 (16) | |
| Characteristics during CAPS event | ||
| Definite CAPS b | 6 (32) | |
| Probable CAPS c | 13 (68) | |
| Time between APS diagnosis and CAPS, months, median (IQR) | 1 (0-36) | |
| Hospitalization in the previous 3 months | 9 (47) | |
| Identified trigger
| 12 (63)
| |
| Clinic characteristics of CAPS | ||
| Peripheral arterial thrombosis | 14 (74) | |
| Peripheral venous thrombosis | 13 (68) | |
| Cardiac involvement | 6 (32) | (50) |
| Pulmonary involvement | 8 (42) | (60) |
| Renal involvement | 8 (42) | (73) |
| CNS involvement | 4 (21) | (56) |
| Cutaneous involvement | 5 (28) | (47) |
| Hematological involvement | 12 (63) | |
| Number of affected organs, median (IQR) | 4 (3-4) | |
| Laboratory Abnormalities | ||
| Lymphopenia d | 12 (63) | |
| Anemia e | 15 (79) | |
| Thrombocytopenia f | 9 (47) | (67) |
| Presence of schistocytes | 3 (16) | (22) |
| Positive Coombs test | 6/11 (55.5) | |
| Low C3 g | 10 (53) | |
| Low C4 h | 12 (63) | |
| CRP levels, median | 6.5 (1.6-9.2) | |
| ESR levels | 24 (6-44) | |
| Positive ANA | 13 (68) | (57) |
| Presence of proteinuria | 11 (58) | |
| Altered prothrombin time i | 13 (68) | |
| Altered partial thromboplastin time j | 9 (47) | |
| Altered INR k | 6 (32) | |
BMI=>30kg/m 2 , b,c according to preliminary classification criteria for CAPS, d Lymphopenia = <1000 X 10^3/µL, e Anemia= <12.5 g/dL, f Thrombocytopenia=<100 X 10^3/µL, g Low C3 =<87mg/dL, h Low C4=<19mg/dL, i Altered prothrombin time =>11.8s, j Altered partial thromboplastin time =>31.2s, k Altered INR =>1.5. Data presented as N (%) unless otherwise indicated.
A Organ and system manifestations, B Instituted treatment.
REFERENCES: NIL.
Acknowledgements: The authors acknowledge all the patients included in the study.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (