

Background: Data regarding the progression of disease and patterns of involved joints in early oligoarticular (oligo) psoriatic arthritis (PsA) is sparse. The FOREMOST study (NCT03747939 [1]) provides a unique opportunity to follow patients with early oligo PsA longitudinally and understand patterns of joint involvement in early oligo PsA. In FOREMOST, apremilast (APR) decreased the number of involved joints vs placebo (PBO) at Week 16 [1].
Objectives: This post hoc analysis of FOREMOST assessed patterns of involved joints at baseline and the effect of APR on different joint groups over time.
Methods: FOREMOST enrolled 308 patients with early oligo PsA (>1 to ≤4 swollen and >1 to ≤4 tender joints; 66–68 joints assessed). Patients were randomized (2:1) to APR (n=203) or PBO (n=105) for 24 weeks (early escape at Week 16), followed by an extension phase in which all patients could receive APR through Week 48. Overall, 291 patients received at least one dose of APR (as randomized, n=203; transitioned from PBO, n=88). Concomitant nonsteroidal anti-inflammatory and/or synthetic disease-modifying antirheumatic drugs were permitted if taken at a stable dose for ≥2 weeks or ≥3 months prior to baseline, respectively, and maintained at the same dose through Week 24. In a post hoc analysis, joints were divided into the groups shown in Table 1. For each group, the percentage of patients (patient incidence) with at least one of the following was calculated at baseline, Week 16, and Week 48: a) swollen and tender joint, b) swollen and/or tender joint, c) swollen joint with no tenderness (“swollen only”), and d) tender joint with no swelling (“tender only”). Baseline and Week 16 data were analyzed for all randomized patients with the last observation carried forward approach for missing data; Week 48 data were analyzed as observed for patients who received at least one dose of APR.
Results: As reported previously, the FOREMOST patient population had low joint involvement at baseline: mean (SD) swollen and tender joint count, 2.6 (0.7) and 3.2 (0.8), respectively [1]. Across all joint groups included in this post hoc analysis, baseline patient incidence of “swollen and tender” and “swollen and/or tender” joints were similar and higher than “swollen only” and “tender only” (Figure 1A). Using the strictest criteria for joint involvement, swollen and tender, the most commonly involved joints at baseline included a mix of large and small joints: finger proximal interphalangeal (PIP) (120/308 [39.0%] patients with at least one involved joint) and metacarpophalangeal (MCP) (102/308 [33.1%]), followed by knee (61/308 [19.8%]), and ankle and tarsus/midfoot (58/308 [18.8%]) (Figure 1A). Baseline patterns of swollen and tender joints were generally similar for APR vs PBO (Figure 1B). Considering the most commonly involved joints, which included large and small joints, greater improvements in swollen and tender finger PIP, MCP, and ankle/tarsus/midfoot joints were observed at Week 16 for APR vs PBO: finger PIP, 23.6% vs 9.5% decrease in patient incidence between baseline and Week 16; MCP, 21.7% vs 13.3% decrease; ankle/tarsus/midfoot, 12.3% vs 8.6% decrease (Figure 1B, C). Baseline incidence of swollen and tender knee joints was higher for APR vs PBO (22.8% vs 14.3%); at Week 16, incidence fell by 16.3% in the APR group vs a 2.9% increase in the PBO group (Figure 1B, C). Across all joint groups, the risk of having ≥1 swollen and tender joint at Week 16 was lower for APR vs PBO (Figure 1D), with statistically significant differences observed for the most commonly involved joints (which included large and small joints): odds ratio (APR vs PBO) (95% CI) for finger PIP, 0.55 (0.31, 0.98); MCP, 0.51 (0.27, 0.97); knee, 0.33 (0.16, 0.71); ankle/tarsus/midfoot, 0.41 (0.17, 0.94) (Figure 1D). The improvements in joint involvement observed with APR at Week 16 were maintained in patients continuing or switching to APR through Week 48 (data not shown).
Conclusion: Our data indicate a mix of large and small joints are involved in early oligo PsA, with hand and finger joints by far most commonly involved. Of note, we observed similar joint patterns as reported at PsA onset in other real-world cohorts [2, 3], strengthening the value of FOREMOST in understanding early oligo PsA. Compared with PBO, APR significantly reduced joint involvement across both large and small joints. Results for joint groups with low patient incidence should be interpreted with caution. Our findings can be helpful to orient physicians when faced with early, oligo PsA.
REFERENCES: [1] Gossec, L et al. Ann Rheum Dis. 2024;83(11):1480-1488.
[2] Zabotti, A et al. RMD Open. 2024;10(2):e004314.
[3] Gladman DD, et al. J Rheumatol. 2021;48(12):1824-1829.
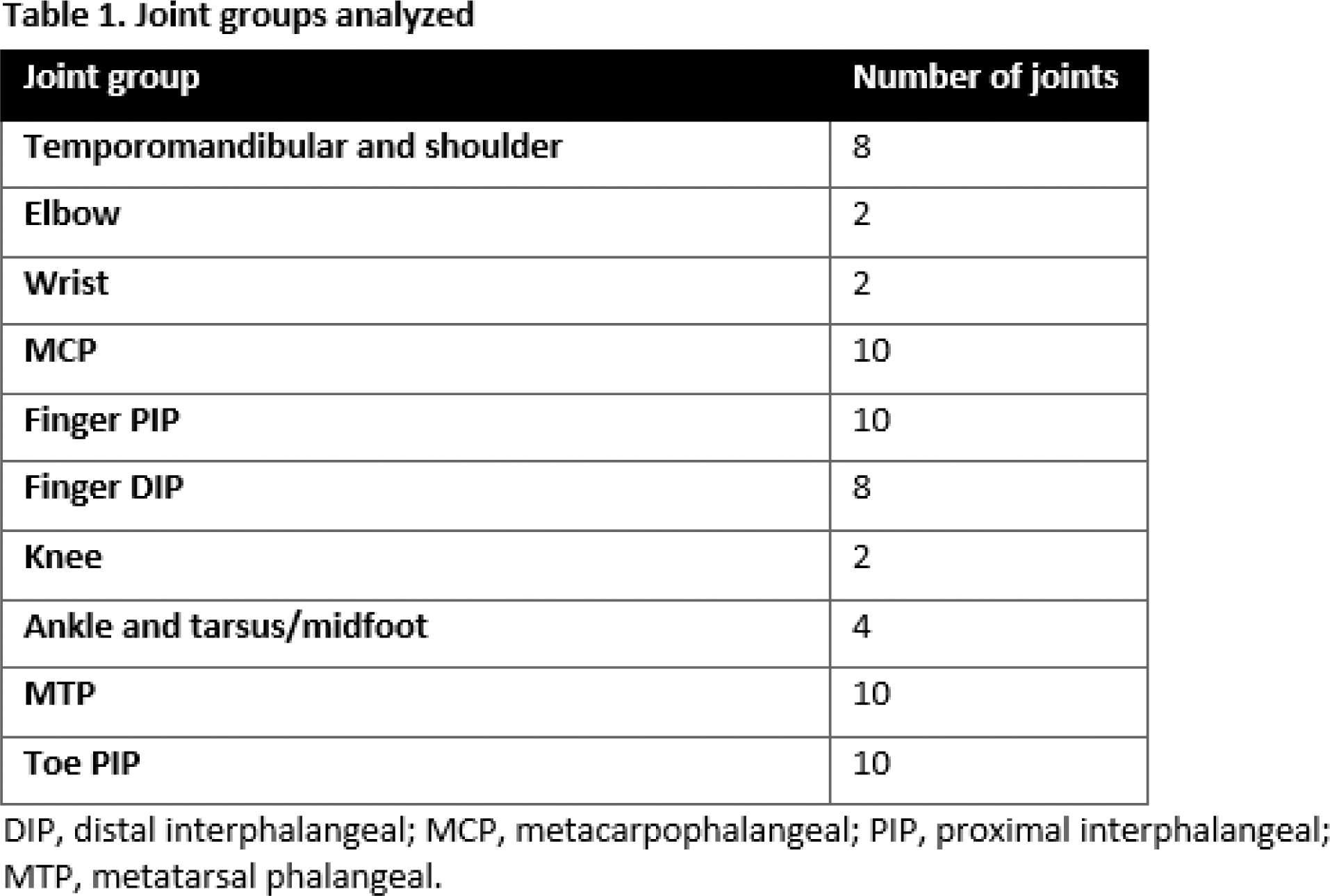
Joint involvement in the FOREMOST study of early oligoarticular PsA.
Acknowledgements: This study was funded by Amgen Inc. Writing support was funded by Amgen Inc. and provided by Rebecca Lane, PhD, of Peloton Advantage, LLC, an OPEN Health company, and Claire Desborough, MSc, CMPP, employee of and stockholder in Amgen Inc.
Disclosure of Interests: Alen Zabotti AbbVie, Amgen, Johnson & Johnson, Lilly, Novartis, UCB, AbbVie, Amgen, Johnson & Johnson, Lilly, Novartis, UCB, Johnson & Johnson, Joseph F Merola AbbVie, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Janssen, Lilly, Moonlake, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB, Ulrich Mrowietz AbbVie, Aditxt, Almirall, Amgen Inc., Aristea, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dr. Reddy’s, Eli Lilly, Foamix, Formycon, Immunic, Janssen, LEO Pharma, Medac, MetrioPharm, Novartis, Phi-Stone, Pierre Fabre, Sanofi-Aventis, and UCB, Philip J. Mease AbbVie, Amgen Inc., Eli Lilly, Janssen, Novartis, Pfizer, UCB, AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sun, UCB, AbbVie, Amgen Inc., Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Sun, UCB, Laure Gossec AbbVie, Amgen Inc., Bristol Myers Squibb, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, UCB, AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Novartis, Pfizer, UCB, MSD, Novartis, Pfizer, Galapagos, AbbVie, Biogen, Lilly, Novartis, UCB, Mitsumasa Kishimoto: None declared , Michele Brunori Amgen Inc., Lichen Teng Amgen Inc., Dafna D. Gladman AbbVie, Amgen Inc., Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, UCB, Laura C. Coates AbbVie, Amgen Inc., Biogen, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Medac, MoonLake, Novartis, Pfizer, UCB, AbbVie, Amgen Inc., Biogen, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Medac, MoonLake, Novartis, Pfizer, UCB, AbbVie, Amgen Inc., Biogen, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Medac, MoonLake, Novartis, Pfizer, UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (