

Background: Systemic lupus erythematosus (SLE) is an autoimmune condition associated with increased cardiovascular (CV) risk, often manifested through subclinical atherosclerosis. This heightened risk is multifactorial, driven by a combination of traditional CV risk factors, chronic systemic inflammation, and autoantibodies. Among these, antiphospholipid (aPL) antibodies, frequently observed in SLE patients, have been implicated in the development of both thrombotic events and structural cardiac abnormalities. Subclinical changes in cardiac function and geometry, such as alterations in left ventricular mass and relative wall thickness, often precede overt cardiovascular disease in these patients. Diastolic dysfunction, a common finding in SLE, may further contribute to impaired cardiovascular outcomes. Despite this, the role of aPL antibodies in mediating these subclinical abnormalities remains unclear, highlighting the need for further investigation.
Objectives: To evaluate the association between the presence of aPL antibodies and alterations in the left ventricle’s geometry, diastolic, and systolic function in SLE patients.
Methods: A cross-sectional and comparative study was conducted on women with SLE, aged 18 years or older, who met the 2019 ACR/EULAR criteria. Patients with prior CV disease, pregnancy, and overlap syndrome were excluded. Patients were divided into two groups according to the presence of aPL antibodies, it was considered positive based on elevated levels of either anticardiolipin or anti-β2-glycoprotein antibodies (>12 U/mL and >20 U/mL, respectively). Echocardiographic measurements were made using B-mode, M-mode, Spectral Doppler, Color Doppler, and Tissue Doppler Imaging. Cardiac geometry, diastolic function, and systolic function were classified according to the 2016 ASE/EACVI criteria. The normality was assessed with the Kolmogorov-Smirnov test. Comparisons were performed using the Student’s T-test, Chi-square test, and Mann-Whitney U test, accordingly. CV risk factors that were related to ventricular geometry and systolic function were included in a multivariable logistic regression. A p-value of ≤0.05 was considered statistically significant.
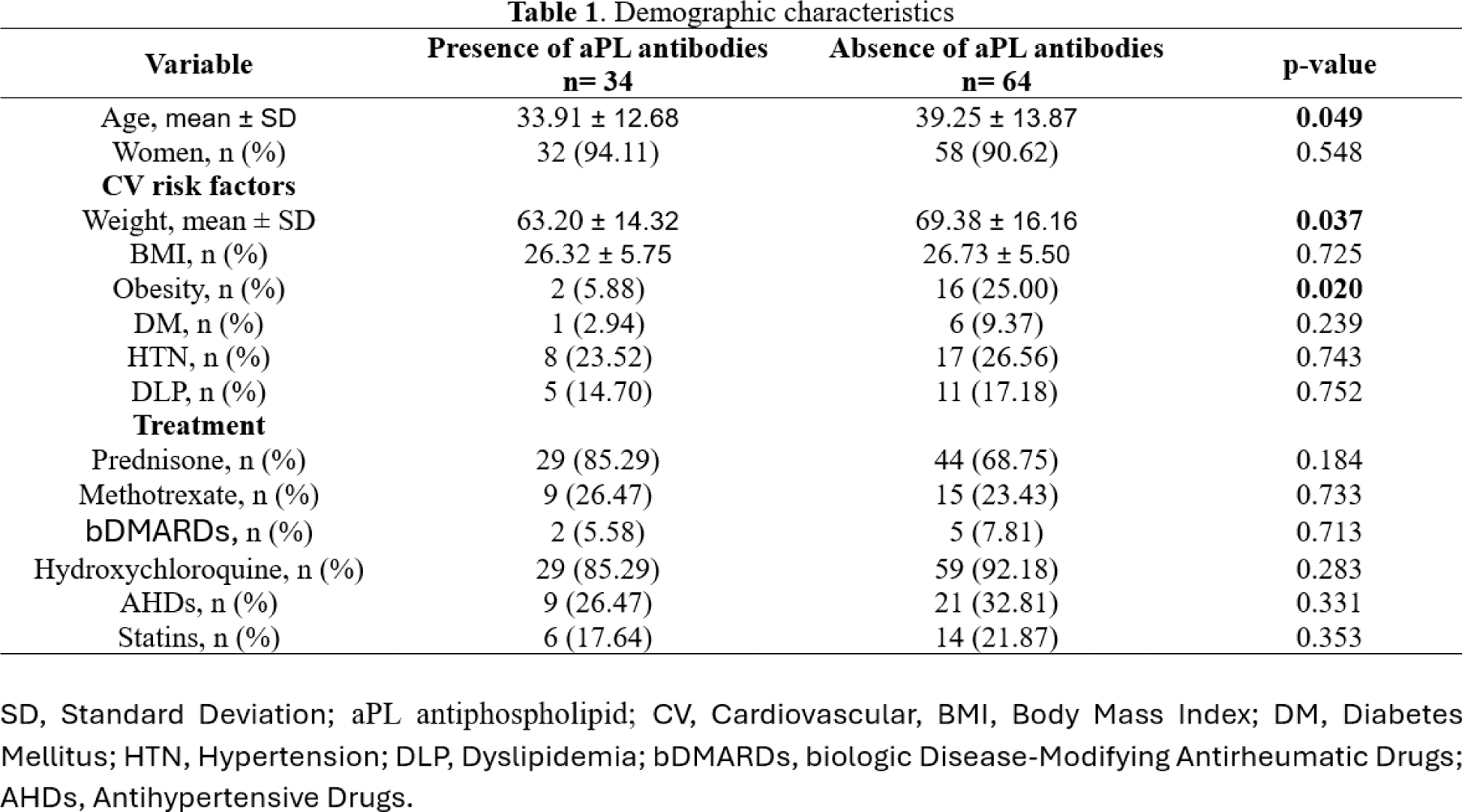
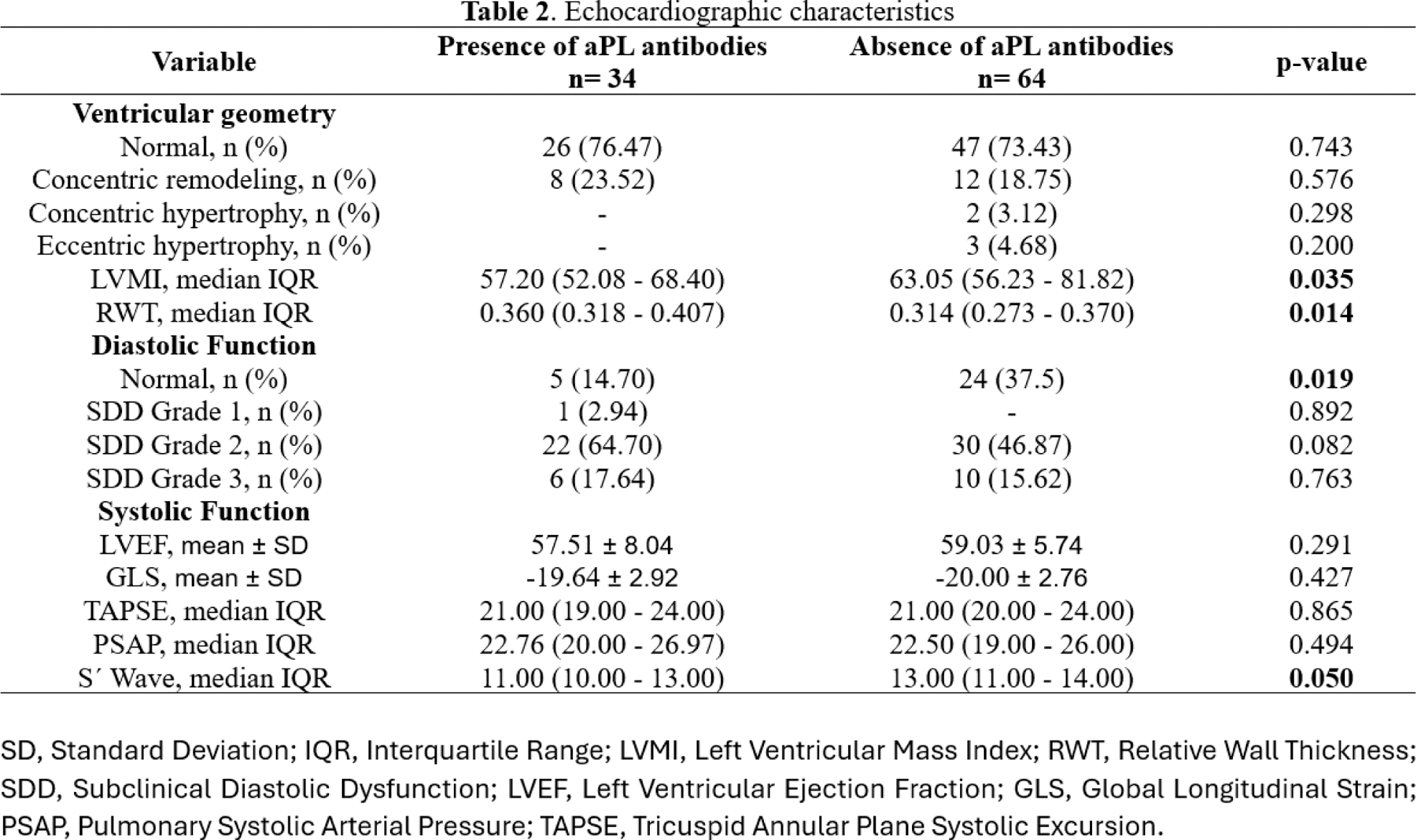
Results: A total of 98 patients were included, with 34 presenting antiphospholipid (aPL) antibodies and 64 without. Patients with aPL antibodies were younger (33.91 ± 12.68 vs. 39.25 ± 13.87 years; p = 0.049) and had lower weight (63.20 ± 14.32 vs. 69.38 ± 16.16 kg; p = 0.037). Obesity was less prevalent in patients with aPL antibodies (5.88% vs. 25.00%; p = 0.020). No significant differences were observed in other traditional CV risk factors or treatment regimens. Patients with aPL antibodies had lower median left ventricular mass index (57.20 vs. 63.05 g/m²; p = 0.035) and relative wall thickness (0.314 vs. 0.360; p = 0.014). To explain this opposite outcome, we performed a multivariable logistic regression which demonstrated that higher weight rates are associated with an increased LVMI value (OR 1.017, 95% CI: 0.800-0.919, p = <0.001). We did the same to clarify the result of eccentric hypertrophy where it was found that higher weight rates are also associated with this variable (OR 1.054, 95% CI: 0.008-0.234, p = 0.037). Diastolic dysfunction was more frequent in patients with aPL antibodies, with fewer patients showing normal diastolic function (14.70% vs. 37.50%; p = 0.019). No significant differences were found in most of the systolic function parameters, including left ventricular ejection fraction (57.51 vs. 59.03; p = 0.291) or global longitudinal strain (-19.64 vs. -20.00; p = 0.427). However, patients with aPL antibodies showed lower S´ Wave values than their counterparts (11.00 vs. 13.00, p = 0.050).
Conclusion: Patients with aPL antibodies had a higher relative wall thickness, as well as higher prevalence of diastolic dysfunction compared to those without aPL antibodies. Despite these findings, no significant differences were observed in systolic function parameters or other traditional cardiovascular risk factors. These results suggest that the presence of aPL antibodies may contribute to early alterations in cardiac geometry and diastolic function, underscoring the importance of targeted cardiovascular assessment in this population.
REFERENCES: [1] Nagy N, Bói B, Papp G, Fiák E, Gáspár-Kiss E, Perge B, et al. Antiphospholipid Antibodies Are Major Risk Factors for Non-Thrombotic Cardiac Complications in Systemic Lupus Erythematosus. Biomedicines. 2024;12(3):530.
[2] Kampolis C, Tektonidou M, Moyssakis I, Tzelepis GE, Moutsopoulos H, Vlachoyiannopoulos PG. Evolution of cardiac dysfunction in patients with antiphospholipid antibodies and/or antiphospholipid syndrome: a 10-year follow-up study. Semin Arthritis Rheum. 2014;43(4):558-65.
[3] Karakasis P, Lefkou E, Pamporis K, Nevras V, Bougioukas KI, Haidich AB, et al. Risk of subclinical atherosclerosis in patients with antiphospholipid syndrome and subjects with antiphospholipid antibody positivity: a systematic review and meta-analysis. Curr Probl Cardiol. 2023;48(6):101672.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (