

Background: Behçet’s syndrome (BS) is a chronic systemic vasculitis in which vascular involvement contributes significantly to morbidity and mortality. For refractory vascular BS (VBS), monoclonal anti-tumor necrosis factor (TNF) antibodies have been recommended beyond glucocorticoids (GCs) and immunosuppressants [1, 2]. However, these recommendations are mostly based on case series or single-arm retrospective studies, with a lack of randomized controlled trials (RCTs) and highly variable duration of efficacy evaluation, limiting the strength of the evidence.
Objectives: We performed the first meta-analysis to evaluate the efficacy and safety of monoclonal anti-TNF antibodies in treating patients with severe and/or refractory VBS at multiple follow-up time points, providing stronger evidence for the formulation of new clinical guidelines.
Methods: This meta-analysis was conducted following the PRISMA guidelines. PubMed, Embase, Cochrane Library, Medline Complete, and Web of Science were systematically searched up to 14 October 2024, using the following strategy: Behçet’s syndrome (all synonyms) AND monoclonal anti-TNF antibodies (all synonyms) AND (‘vascular’ OR ‘vasculo’ OR ‘vasculitis’). Therapeutic efficacy was evaluated by the degree of clinical and imaging remission. Specifically, clinical response was categorized as a) complete remission (CR): resolution of VBS-related symptoms; b) partial remission (PR): improvement in VBS-related symptoms; c) no response (NR): failure to achieve CR or PR [3]. Imaging remission was defined as no worsening or new findings compared to baseline, including improvement [4]. Data from eligible studies were used to calculate the pooled rates of clinical remission at 3, 6, and 12 months, along with subgroup analysis based on the specific type of anti-TNF agents, including infliximab, adalimumab, and golimumab. Pooled proportions of imaging remission before and after 6 months were also performed. Adverse drug reactions were recorded in detail. The results were synthesized by a randomized effects model using R platform and presented as forest plots with 95% confidence intervals.
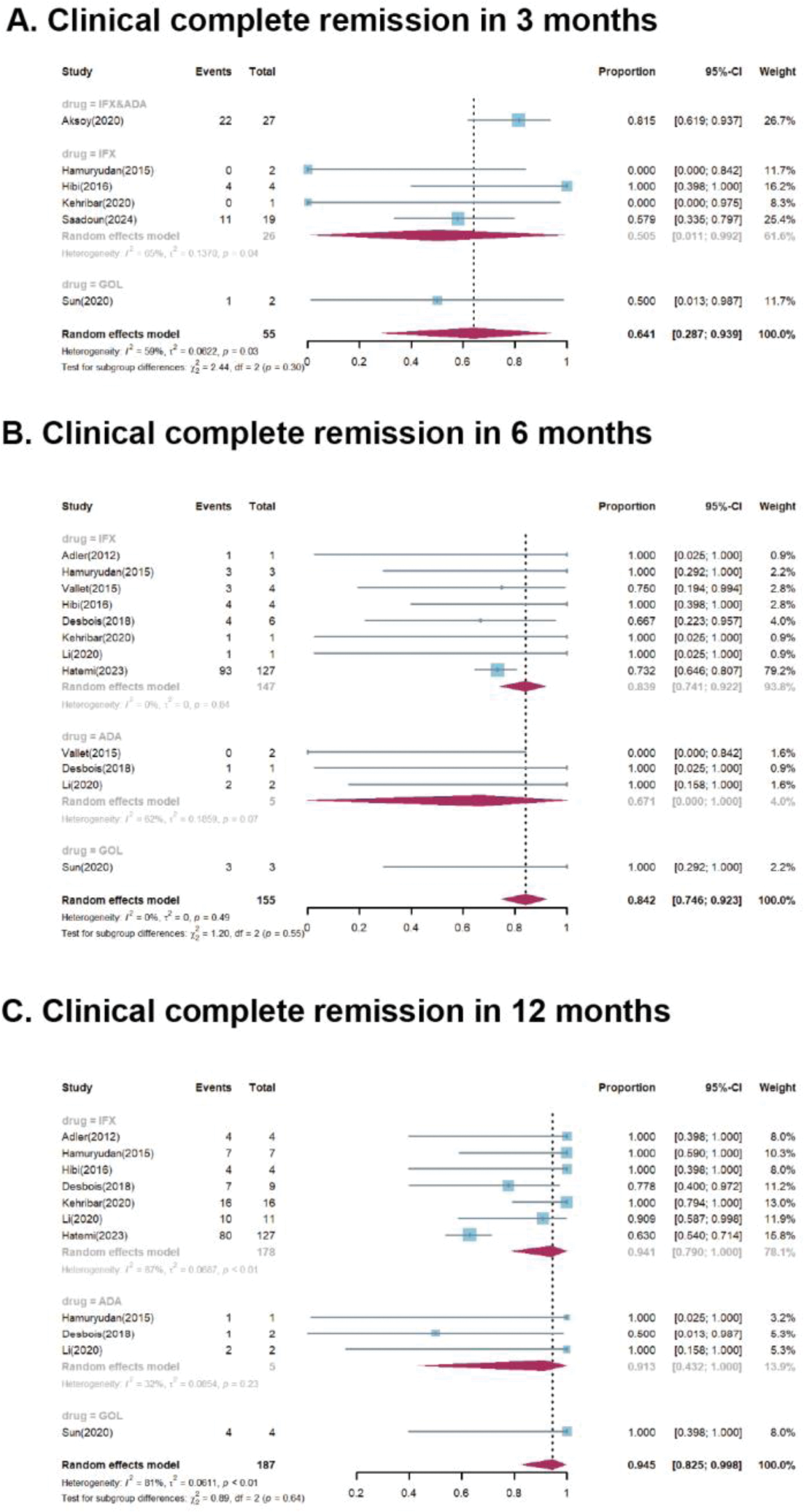
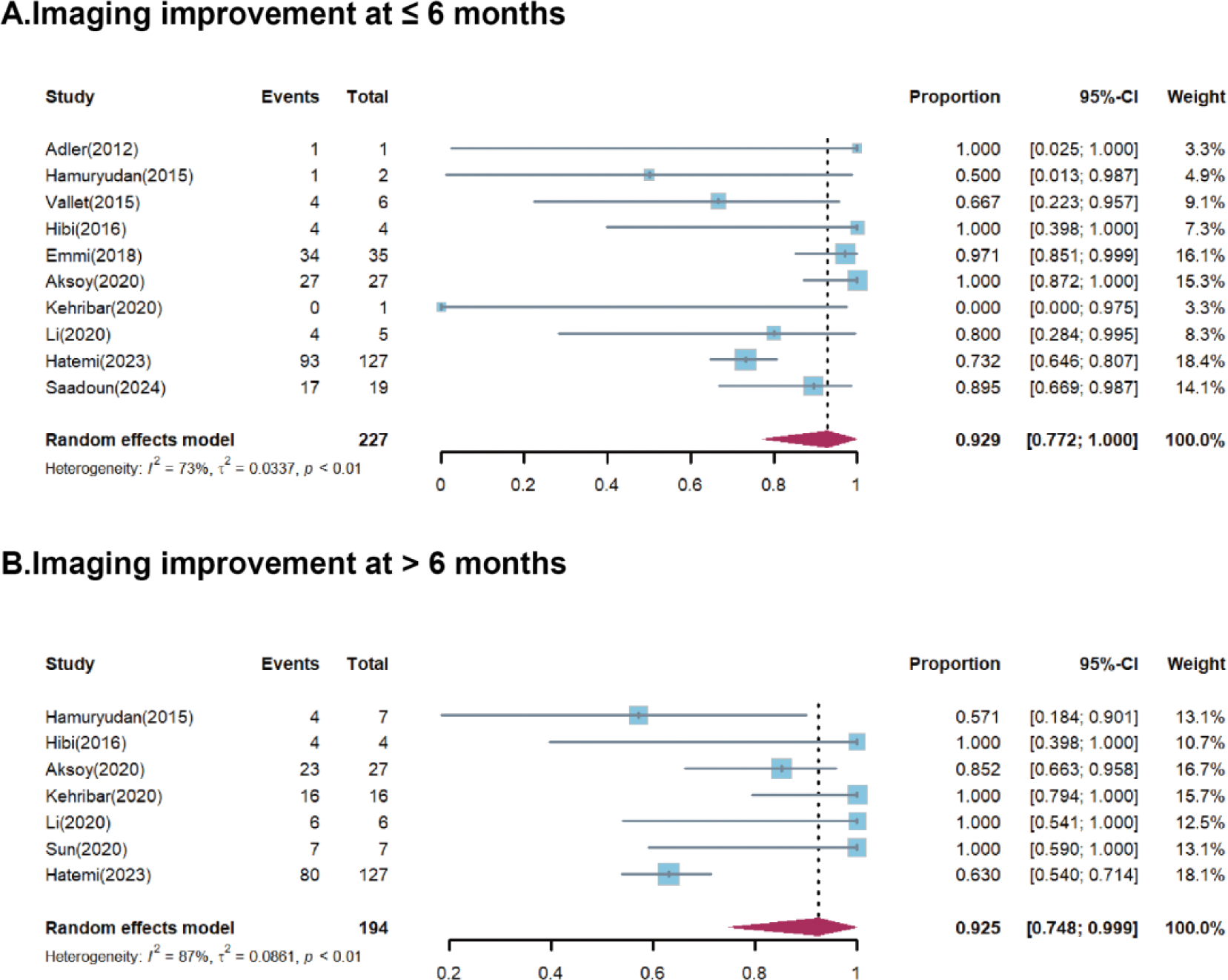
Results: Twelve studies were identified, including 1 randomized controlled trial, 10 single-arm cohort studies, and 1 case series. Of the 297 VBS patients included, 163 had venous involvement, 86 had arterial involvement, and 48 had both. Two patients were treated with anti-TNF antibodies as the first-line therapy, while others had a history of relapse, refractoriness, or intolerance to previous conventional treatment. The pooled proportions of CR were 64.1% (95%CI 28.7-93.9%), 84.2% (95%CI 74.6-92.3%), and 94.5% (95%CI 82.5-99.8%) at 3, 6, and 12 months, respectively, with corresponding I 2 values of 59%, 0%, and 81% (Figure 1). Clinical PR was achieved in 77.7% (95%CI 28.9-100%) of patients at month 3, increasing to 95.0% (95%CI 82.4-100%) and 96.0% (95%CI 85.6-100%) at month 6 and 12, respectively. Imaging improvements were observed in 92.9% (95% CI 77.2-100%) of patients within the first six months (Figure 2). During monoclonal anti-TNF antibodies treatment, 26 patients were reported to have relapsed. Among the 43 patients who stopped treatment due to remission, 12 patients relapsed. Adverse events were reported in 9 studies involving 42 patients, 31 of whom experienced severe AEs, with 5 deaths related AEs.
Conclusion: Monoclonal anti-TNF antibodies are an effective therapy with good clinical and radiological efficacy and low AEs for VBS. Prevention of relapses and control of disease progression remain critical goals in managing VBS. To further validate their efficacy, RCTs stratified by arterial and venous involvement are warranted.
REFERENCES: [1] Hatemi G. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis 2018;77:808–18.
[2] Moriano Morales C, Graña Gil J, Brito García N, Martín Varillas JL, Calvo Del Río V, Moya Alvarado P, et al. SER recommendations on treatment of refractory Behçet’s syndrome. Reumatol Clin (Engl Ed) 2024;20:204–17.
[3] Vallet H, Riviere S, Sanna A, Deroux A, Moulis G, Addimanda O, et al. Efficacy of anti-TNF alpha in severe and/or refractory Behçet’s disease: Multicenter study of 124 patients. Journal of Autoimmunity 2015;62:67–74.
[4] Hatemi G, Tukek NB, Esatoglu SN, Ozguler Y, Taflan SS, Uygunoglu U, et al. Infliximab for vascular involvement in Behçet’s syndrome. Clinical Immunology 2023;253:109682.
Forest plots for subgroup analysis of clinical complete remission after specific type of anti-TNF agent treatment in 3 months (A), 6 months (B), and 12 months (C). ADA, adalimumab; CI, confidence interval; IFX, infliximab; GOL, golimumab
Forest plots for marked imaging remission after anti-TNF agent treatment at ≤ 6 months (A), and > 6 months (B).
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (