

Background: The medical app Axia is a novel digital therapeutic for axial spondyloarthritis (axSpA) compliant with the European medical device regulation (MDR). Axia provides an innovative app-based intervention for axSpA patients, integrating tailored home-based exercise therapy, patient education and comprehensive disease management.
Objectives: We aim to evaluate the efficacy of Axia in a randomized controlled interventional trial (RCT) among German axSpA patients conducted over 12 weeks, with an initial analysis at 8 weeks.
Methods: In this 12-week RCT, 200 axSpA patients on stable pharmacotherapy were randomized (1:1) to using Axia for 12 weeks (intervention group) vs. standard care (control group). The primary endpoints are improvement in disease activity (Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)), disease-specific functionality (BASFI (Bath Ankylosing Spondylitis Functional Index)) and disease-specific quality of life (ASQoL (Ankylosing Spondylitis Quality of Life)) after 12 weeks. An analysis after 8 weeks was conducted on the first 30 participants in each group.
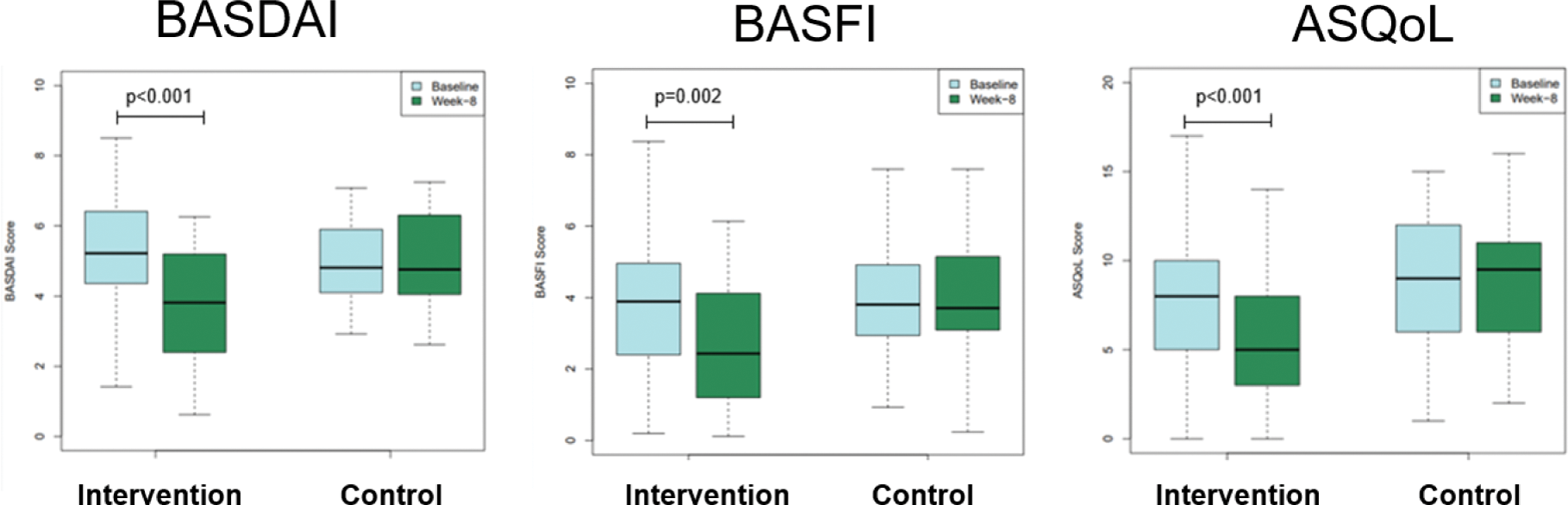
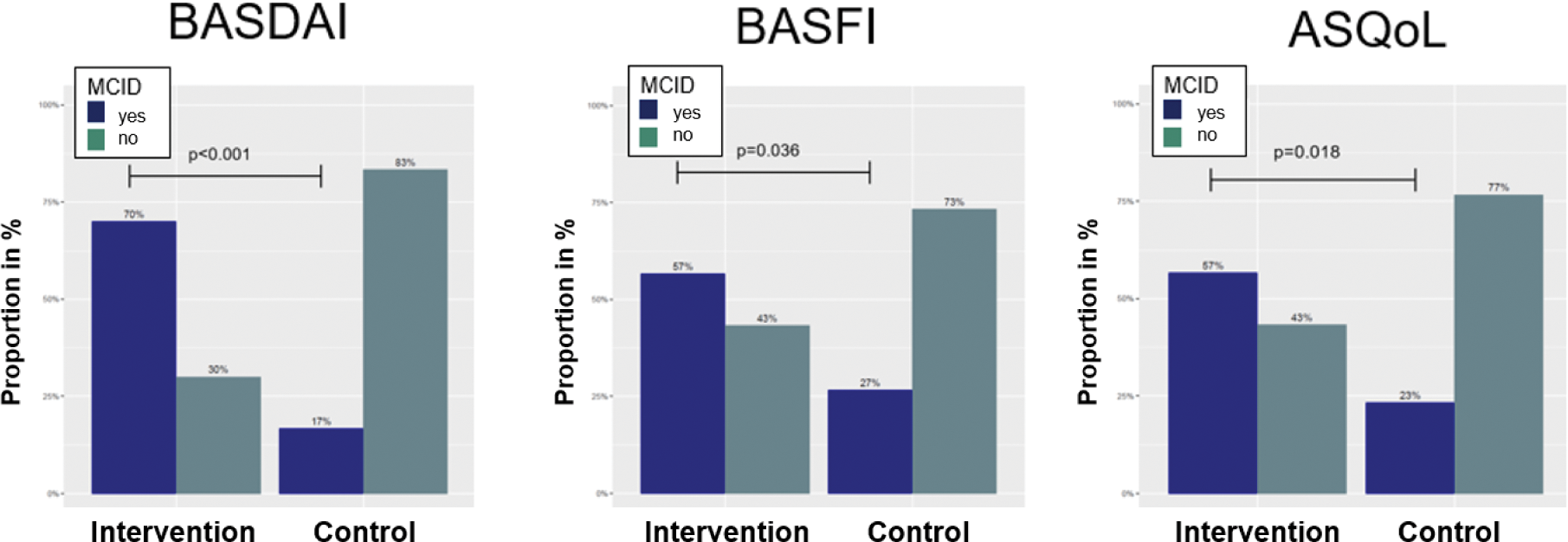
Results: At week 8, participants in the Axia intervention group presented significant improvements in BASDAI (-1.60 [SD: 1.31] vs. control: -0.07 [1.25], p<0.001), BASFI (-0.80 [1.27] vs. control: -0.01 [1.11], p=0.004) and ASQoL (-1.93 [2.80] vs. control: +0.17 [2.57], p<0.001) while the control group showed no improvement (Figure 1). Furthermore, more patients receiving Axia reached a minimal clinically important difference (MCID) improvement in BASDAI (Axia: 70% vs. control: 17%, p<0.001), BASFI (Axia: 57% vs. control: 27%, p=0.036) and ASQoL (Axia: 57% vs. control: 23%, p=0.018) than patients of the control group (Figure 2). No concerning safety signals were observed.
Conclusion: In this 8-week analysis of a RCT of axSpA patients on stable pharmacotherapy, the digital therapeutic Axia was able to significantly improve diseases activity, functionality, and quality of life among the first 30 participants of the intervention group compared to the 30 participants of the control group.
REFERENCES: NIL.
Trial registration: DRKS.de, DRKS00033783. Registered on 01 Mar 2024.
The intervention group showed improvements in mean BASDAI, BASFI and ASQoL after 8 weeks
ASQoL, Anyklosing Spondylitis Quality of Life; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Function Index.
More participants in the intervention group reached minimal clinically important difference improvement
ASQoL, Anyklosing Spondylitis Quality of Life; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Function Index.
Acknowledgements: NIL.
Disclosure of Interests: Patrick-Pascal Strunz Chugai (25000€), Abbvie (12000€), Tobias Heusinger Applimeda GmbH, Maxime Le Maire Applimeda GmbH, Anna Fleischer: None declared, Karsten Sebastian Luetkens: None declared, Patricia Possler: None declared, Michael Gernert: None declared, Hannah Labinsky: None declared, Robert Leppich Applimeda GmbH, Astrid Schmieder: None declared, Ludwig Hammel: None declared, Billy Sperlich: None declared, Matthias Fröhlich: None declared, Marc Schmalzing: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (