

Background: Interstitial lung diseases (ILD) are severe manifestations of systemic autoimmune diseases (SARD) that are associated with an increased mortality. Though the primary endpoint was not met, the focuSSed randomized controlled trial suggested that tocilizumab might preserve lung function in patients with systemic sclerosis (SSc) associated ILD. Similar results were observed in retrospective studies with small sample size for rheumatoid arthritis (RA) associated ILD.
Objectives: Our aim was to assess the effectiveness and tolerability of interleukin-6 inhibitors (IL6i) in SARD-ILD using a real-world cohort.
Methods: In this retrospective cohort study, we used an electronic query at a tertiary hospital to identify patients with one code for ILD and one code for either SSc, RA, Sjogren Syndrome (SS), inflammatory myositis (IM), systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD) or undifferentiated connective tissue disease (UCTD) initiating an IL-6i. The diagnosis of SARD was confirmed by electronic medical record review according to corresponding EULAR/ACR classification criteria. ILD diagnosis was validated by a multidisciplinary team including an expert thoracic radiologist who reviewed the chest high-resolution computed tomography (HRCT) scans. We collected data regarding pulmonary function tests (PFTs), change in ILD extension on HRCT, lung transplant free-survival, and adverse events by chart review. A linear mixed model with random intercept was used to compare the within-patient trajectory of the percent predicted and crude forced vital capacity (FVCpp and FVC, respectively) and percent predicted diffusing capacity of carbon monoxide (DLCOpp) within 18-months before to 18-months after IL6i introduction.
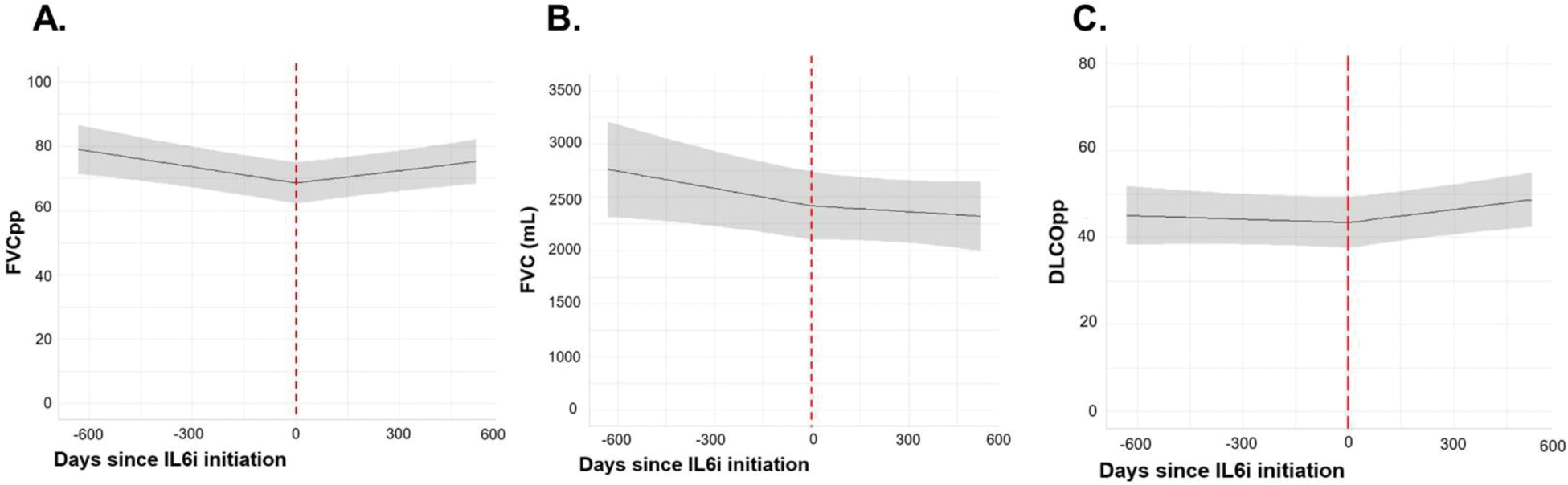
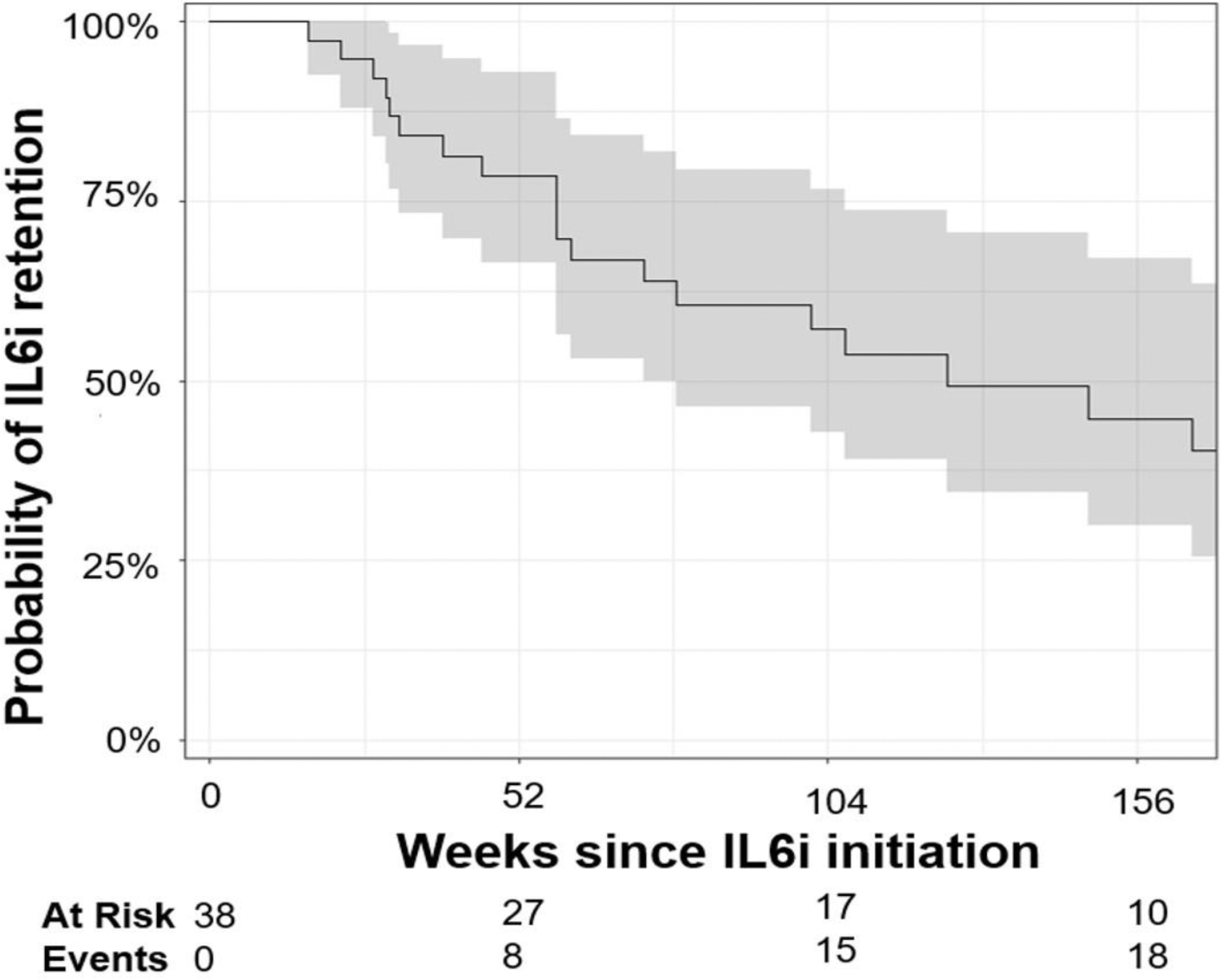
Results: We included 38 patients with SARD-ILD initiating an IL6i, (mean age 58.4 years, 11 males, 12 with MCTD, 8 with SSc, 6 with RA, 6 with UCTD, 4 with IM, and 2 with a primary SS, Table 1). Mean FVCpp and DLCOpp at IL6i initiation were 74.7 ± 19.4, and 45.8 ± 16.7, respectively. We observed a significant change in the trajectory of FVCpp (-7.3% per year before IL6i initiation vs. +2.9% per year after, p = 0.002), FVC (- 178 mL per year before IL6i initiation vs. -82,8 mL per year after, p=0.048), and DLCOpp (-0.17% per year before IL6i initiation vs +4.7% per year after, p = 0.02), Figure 1. After a mean follow up duration of 57.4 ± 26.1 weeks, clinical reports of HRCT chest scans indicated that ILD extension was stable in 19/34 patients, regressed in 5/34, and progressed in 10/34. At the end of follow-up, 18/38 patients had stopped IL6i with a median drug retention of 124.0 weeks, 95% CI (73.1, 330.0), Figure 2. Reasons for cessation included ILD progression (n=6), ILD remission (n=2), patient’s own choice (n=2), infection (n=2), relapse of articular disease (n=2), death (n=2), pregnancy intention (n=1), pulmonary hypertension (n=1). While receiving IL6i, adverse events were reported in 8/38 patients: 3 severe infections, 2 non-severe infections, 2 cancers, 2 deaths and 1 transaminitis.
Conclusion: In this real-world study of IL6i in SARD-ILD, a significant improvement of lung function was observed after IL6i initiation. In good agreement, following IL6i treatment, stability or regression of ILD was observed in more than 60%. Further research is needed to confirm the direct implication of IL6i in our results.
Patient characteristics at interleukin-6 inhibitors initiation
| Patients
|
|
|---|---|
| Female sex, n (%) | 27 (71) |
| Mean age, years (SD) | 58.4 (14.3) |
| Current tobacco smokers, n (%) | 2 (5) |
| SARD, n (%) | |
| - SSc | 8 (21) |
| - AS | 4 (11) |
| - MCTD | 12 (32) |
| - UCTD | 6 (16) |
| - RA | 6 (16) |
| - SS | 2 (5) |
| ILD pattern, n (%) | |
| - Fibrotic NSIP | 21 (55) |
| - Cellular NSIP | 4 (10) |
| - UIP | 8 (21) |
| - OP | 3 (7.9) |
| - NSIP/OP | 2 (5) |
| Mean FVCpp, (SD) | 68.8 (19.4) |
| Mean DLCOpp, (SD) | 45.8 (16.7) |
| Concomitant daily dose of steroids in equivalent prednisone, n (%) | |
| - No concomitant steroids | 11 (29) |
| - 10 mg or less | 15 (39) |
| - More than 10 mg | 12 (32) |
| Concomitant use of antifibrotics, n (%) | 6 (15.7) |
Trajectories of FVCpp (panel A), FVC (panel B) and DLCOpp (panel C) before and after interleukin-6-inhibitor initiation. The shaded grey represents the 95%CI of the trajectory. FVC: forced vital capacity, DLCO: diffusing capacity of carbon monoxide, IL6i: interleukin-6 inhibitor.
Kaplan-Meier curve for retention of interleukin-6 inhibitor. The shaded grey represents the 95%CI of the trajectory.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Anne Dupont: None declared, Raphael Borie Roche Boehringer Ingelheim Savara, Clairelyne Dupin: None declared, Esther Ebstein: None declared, Sebastien Ottaviani: None declared, Marine Forien: None declared, Pierre Leguen: None declared, Quentin Philippot: None declared, Bruno Crestani BMS AstraZeneca CSL Behring GSK Roche Sanofi Boehringer Ingelheim, Philippe Dieudé BMS Lilly Pfizer Medac Novartis UCB Pharma Boehringer Ingelheim AbbVie, Pierre-Antoine Juge Boehringer Ingelheim Novartis, Galapagos.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (