

Background: Subcutaneous (SC) infliximab (IFX) is a recent alternative to the intravenous (IV) formulation for the treatment of chronic inflammatory rheumatic diseases (IRDs). While its therapeutic equivalence has been demonstrated for labeling purposes, no real-world data are available in rheumatology regarding the outcomes of switching from IV to SC formulation.
Objectives: The primary objective of this study was to evaluate the drug retention rate of SC IFX after transitioning from IV IFX. Secondary objectives included assessing the acceptability of the switch, the tolerability of the SC formulation, patient satisfaction, and the cost impact of the switch.
Methods: We performed a prospective, single-center, observational study. Adult patients with IRDs in remission who had been receiving IV IFX at standard doses (3–5 mg/kg every 6–8 weeks) for at least three infusions were eligible for inclusion. The switch was first proposed to the treating physician and, upon their agreement, to the patient. Patients included in the study were assessed at the time of the switch and at 3-, 6-, and 12-months of follow-up. Clinical, biological, and questionnaire data (including patient’s satisfaction with the SC formulation) were collected at each visit. Serum infliximab and anti-infliximab antibody levels were measured at baseline and after 6 and 12 months. For patients switched by their treating rheumatologist outside of the study, the 1-year drug retention rate was recorded. The direct cost impact of the switch was estimated by comparing treatment-related costs (including cost of treatment, day hospitalizations and consultations) during the year prior to and the year following the switch.
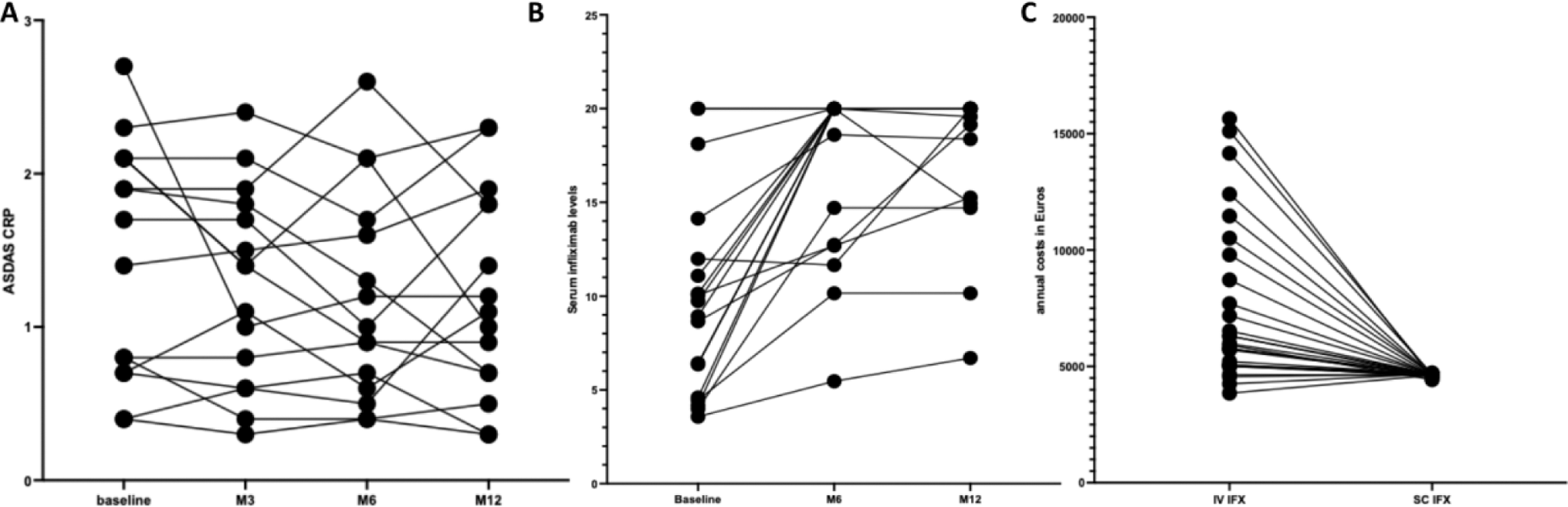
Results: Of the 173 patients on IV IFX as of January 1, 2023, 88 were eligible for the study. The main reason for non-inclusion was the absence of a standard dose (> 3-5mg/kg or interval between 2 infusions > 8 weeks) for 62 patients. Physician approval was obtained for 52% (n = 46), but 14 patients declined the switch and 10 others patients switched outside the study. The main reasons for physician refusal were concerns about nonadherence (40%) and difficult to manage patients (28%). Patients who refused the switch mainly preferred to visit the hospital rather than managing treatment at home (n = 9). Twenty-two patients were finally included in the study: 16 with axial spondyloarthritis, 5 with psoriatic arthritis, and 1 with an unclassified IRD. Patients had a mean age of 56 ± 17 years and 45% were female. As required, the disease was in remission at inclusion, with a mean ASDAS-CRP of 1.6 ± 0.9 and a DAS28-CRP of 2 ± 0.4. One-third of patients were treated with IFX as their first-line biologic, with an average treatment duration of 8.9±5years, corresponding to 63±39 IV infusions. Additionally, 50% of patients were co-treated with methotrexate. At 6 and 12 months, 87% (19/22) of patients maintained SC IFX with stable disease (Figure 1A). SC injections were discontinued in 3/22 patients after 2–3 injections due to intolerance (n = 2: nausea and vomiting in one patient; recurrent sinusitis/sore throat in another) and subjective loss of efficacy (n = 1). Regarding safety, 6 patients reported mild adverse effects (pruritus: n = 1; injection pain: n = 2; injection site reactions: n = 3), and all but one resolved by the end of the study. Mean pre-dose serum infliximab levels increased from 10.3 ± 5.6 µg/ml to 17 ± 6 µg/ml after switching to SC IFX (Figure 1B). During the follow up, no patients developed anti-drug antibodies. Overall satisfaction with SC IFX was high, with a mean of 9.7 ± 0.37 out of 10. Most of patients (n = 17) highlighted time saving as a key positive aspect and the majority of patients appreciated the increased flexibility to adapt to various circumstances, such as travel or illness. In parallel, 12 patients switched to SC IFX by their treating rheumatologist in routine care without participating to the full study. Within the whole combined populations of patients (n = 22 + 12 = 34), retention rate at 12 months was 85%. Of major interest, the annual mean cost of treatment per patient significantly decreased with the SC formulation (€4630 ± 22) compared to IV (€7393 ± 1288, p < 0.001), primarily due to reduced costs associated for hospital admissions for infusions (Figure 1C).
Conclusion: These results confirm the efficacy for maintaining remission, good tolerability, and economic benefits of switching to SC IFX in well-controlled patients previously receiving IV IFX. The SC formulation represents an attractive option to optimize the management of IRDs while reducing costs associated with hospital-based care with a high patient satisfaction.
Changes in ASDAS (A) and infliximab levels (B) in the year after switching from IV to SC formulation. Representation of direct cost with the IV and SC formulation. Each line composed of 1 to 4 points represents one patient.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (