

Background: The Study of Antimalarials in Incomplete Lupus Erythematosus (SMILE, NCT03030118) was a double-blind, randomized, placebo-controlled study of hydroxychloroquine (HCQ) to prevent the development of lupus in people at risk. Participants were females and males aged 15-49 who had an ANA of ≥1:80 and one or two but not three 2012 SLICC criteria for the classification of systemic lupus erythematosus. They were randomized to either 400 mg/d of HCQ (200 mg for participants under 40kg) or matching placebo (PBO). They were followed for up to 24 months for the development of new SLICC criteria. 187 individuals were enrolled in the trial. In addition to SLICC criteria, biospecimens were collected every three months for several mechanistic studies including genomic, proteomic, and transcriptomic studies. One goal of the mechanistic studies is to understand the effect of HCQ on features on autoimmunity in the SMILE participants.
Objectives: Assess the effect of hydroxychloroquine on the level of antibodies to a panel of 120 potential autoantigens.
Methods: Serum was collected from SMILE participants at baseline and every three months for a total of 24 months or until they met SLICC classification criteria for SLE. IgG and IgM antibodies to 120 antigens previously demonstrated in patients with SLE and other autoimmune diseases were quantified by fluorescence intensity on antigen microarrays [1, 2]. Signal intensity was normalized to total IgG for each sample and log 2 transformed for analysis. Levels of individual autoantibodies were compared at baseline and at the last study visit and significance determined using a paired t-test.
Results: 152 of the 187 SMILE participants had informative pairs of serum samples. These included 46 individuals who got HCQ and 50 who got PBO and did not develop new SLICC criteria over 24 months (“Non-Progressors”, NP); 16 individuals who got HCQ and 16 who got PBO who developed a second SLICC criterion in addition to the ANA (“Progressors”, PR; 12 individuals who got HCQ and 12 who got PBO who developed criteria for classification of lupus (“Developed SLE”, DS).The Progressors and Developed SLE individuals were combined (PRDS) for comparison to the NP. At a nominal significance of p<0.05, the PRDS-HCQ showed significant decreases in 86 of 120 autoantibodies while in the PRDS-PBO cohort, only 17 of 120 autoantibodies had significant decreases. The effect was qualitatively similar in the NP-HCQ cohort where responses were decreased for 71 of 120 antigens and for NP-PBO where responses were decreased for 36 of 120 antigens. On average, antibody concentrations decreased 21.4% in PRDS-HCQ participants and 6.2% in PRDS-PBO participants. Responses to individual antigens varied. For example, antibodies to genomic DNA decreased 53% in HCQ treated participants and 25% in PBO treated participants; anti-nucleolin decreased 33% in HCQ treated participants and 2% in PBO treated participants (Figure 1); anti-Sm decreased 16% in HCQ-treated participants and 7% in PBO treated participants (not shown). After correcting for multiple comparisons, responses to 9 antigens showed significant decreases in the HCQ-treated individuals. These included aquaporin 4, GP210, IA-2, lysozyme, MCP-1, Mi-2, nucleolin, PL-7, and thyroglobulin. Decreases in the HCQ-treated individuals ranged from 20%-34% versus 0% to 16% in PBO-treated individuals.
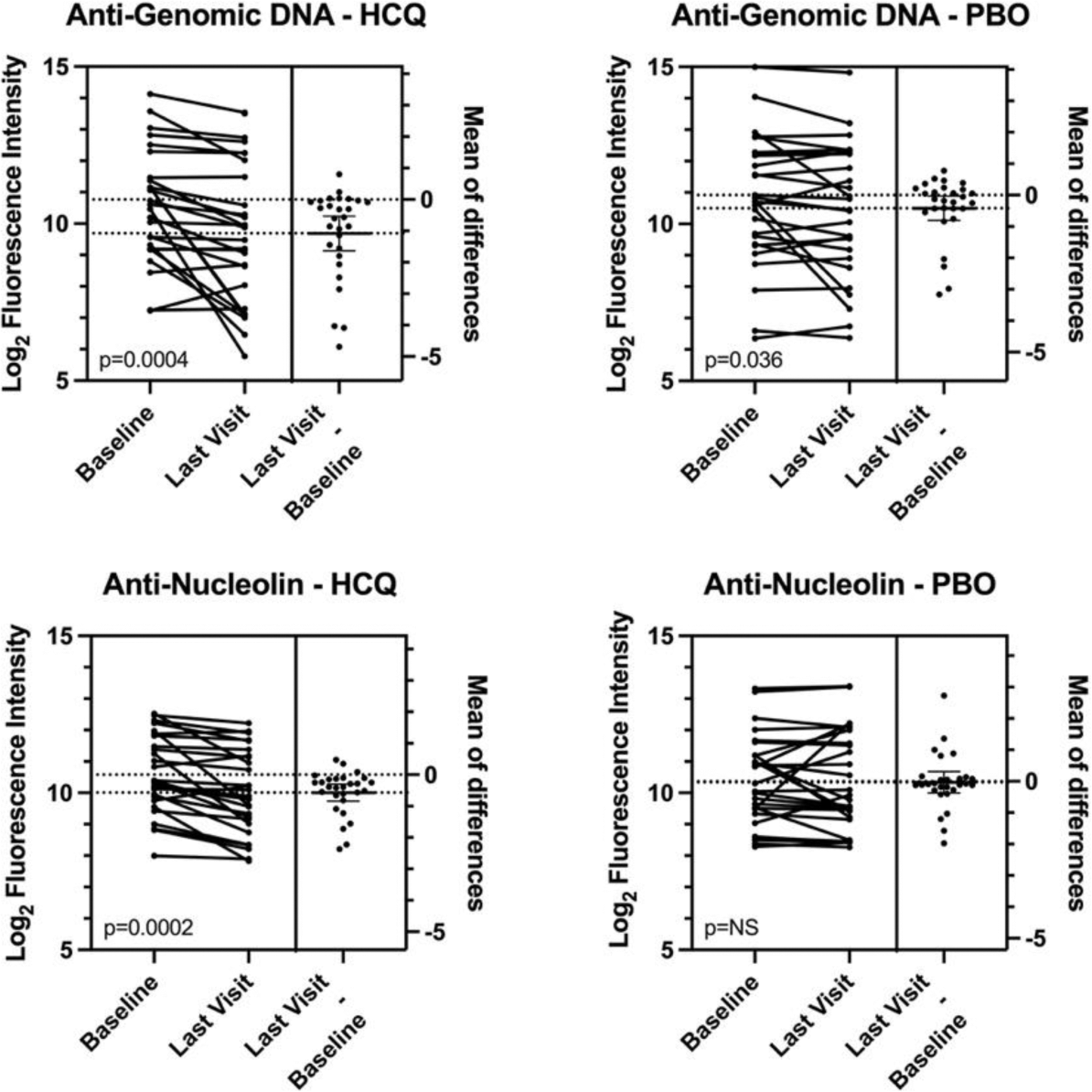
Conclusion: In a cohort of individuals at risk for the development of systemic lupus erythematosus, treatment with hydroxychloroquine resulted in significant decreases in autoantibody responses to a diverse panel of antigens. This feature of treatment with hydroxychloroquine may be a factor in the clinical efficacy of this agent.
REFERENCES: [1] Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol (2007) 147(1):60-70. Epub 2006/12/21. doi: 10.1111/j.1365-2249.2006.03251.x.
[2] Olsen NJ, Li QZ, Quan J, Wang L, Mutwally A, Karp DR. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther (2012) 14(4):R174. Epub 2012/07/31. doi: 10.1186/ar3927.
Acknowledgements: This work was supported by NIH Grants U01 AR071077 and U01 AI176135.
Disclosure of Interests: David Karp DSMB for Sanofi, Zura, and Caribou, Institutional support from BMS, Roche, UCB, Eli Lilly, Novartis, Biogen, Prithvi Raj: None declared, Chengsong Zhu: None declared, Duanping Liao: None declared, Dajiang Liu: None declared, Nancy Olsen: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (