

Background: Patients with rheumatoid arthritis (RA) treated with Janus Kinase inhibitors (JAKi) are at increased risk of Herpes Zoster (HZ) [1, 2]. A non-live subunit vaccine against HZ (HZ/su), consisting of Varicella Zoster virus (VZV) glycoprotein E (gE) and an adjuvant system, has been shown to induce antibody and T-cell responses in healthy adults ≥50 years of age [3]. We previously showed that for patients with RA receiving JAKi, the HZ/su vaccine was safe and induced serologic responses with increased anti-gE antibody levels, although lower than in healthy controls [4]. Cellular immune responses are believed to play an important role in the defence against HZ [5], but data on cellular immunogenicity of the HZ/su vaccine for this population is limited [6].
Objectives: To further investigate cellular immune responses to the HZ/su vaccine among patients with RA treated with JAKi, as compared to healthy controls. In addition, cellular immune responses were explored in patients who developed HZ after the vaccination.
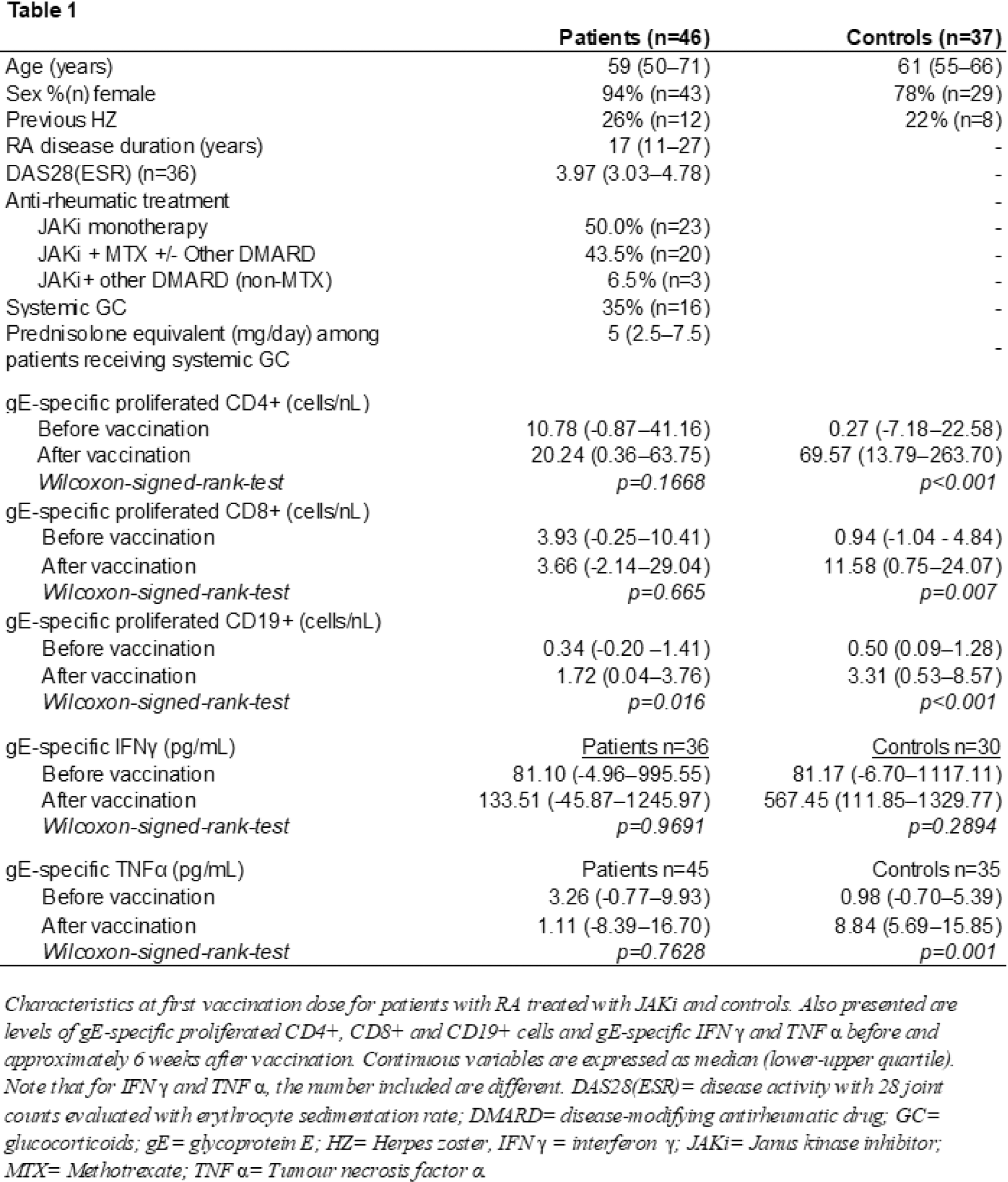
Methods: RA patients treated with JAKi (n=82) at the Department of Rheumatology, Skåne University Hospital, Sweden, and healthy controls (n=51) received two doses of the HZ/su vaccine, as previously described (4). Blood samples were collected before and approximately 6 weeks after vaccination. Peripheral blood mononuclear cells (PBMCs) were isolated and frozen in liquid nitrogen. After rapid thawing, PBMCs were prepared and stained with CellTrace Violet and cultured for 7 days with VZV gE (gE-stimulated) and without stimulation (negative control). The concentration of proinflammatory cytokines TNFα and IFNγ was analysed in supernatants from the gE-stimulated and unstimulated samples using an immunoassay. Proliferation of CD4+, CD8+ and CD19+ cells were assessed by flow cytometry. gE-specific proliferation and cytokine production were compared before and after vaccination using Wilcoxon Signed-Rank test.
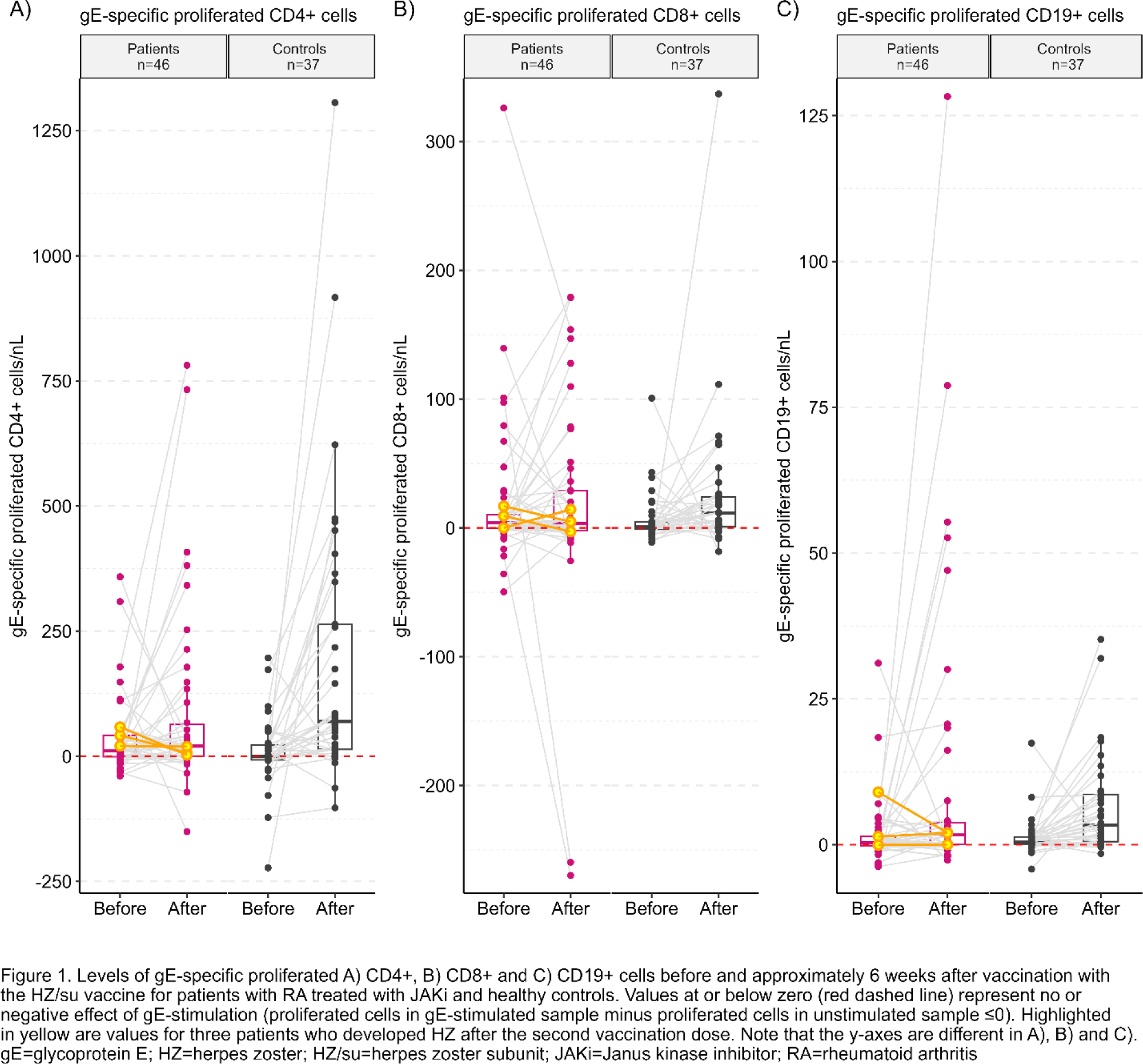
Results: Cellular proliferation data was assessable for 46 patients and 37 controls (Table 1). Following vaccination, gE-specific proliferation of CD19+ cells increased significantly among patients (p=0.016) and controls (p<0.001) (Figure 1). For patients, there were no statistically significant differences in gE-specific proliferation of CD4+ or CD8+ cells, whereas controls responded with significant increases. After vaccination, gE-specific production of TNFα (assessable for n=45 patients and n=35 controls) increased significantly for controls, but no statistically significant difference was seen for patients. For IFNγ (assessable for n=36 patients and n=30 controls), no statistically significant differences were seen for patients or controls. Of 7 patients who developed HZ after the vaccination, cellular proliferation was assessable in three. No or low increase in gE-specific cell proliferation of CD4+ and CD19+ cells were seen for these three patients after vaccination (highlighted in Figure 1), with similar results for TNFα (assessable for n=3) and IFNγ (assessable for n=2). One of these 3 patients had an increase in gE-specific proliferation of CD8+cells of similar magnitude as the controls.
Conclusion: After two doses of the HZ/su vaccine, patients with RA treated with JAKi responded with significantly increased gE-specific proliferation of CD19+ cells. However, the absence of significant increases in gE-specific proliferation of CD4+ and CD8+ T cells and proinflammatory cytokines indicate impaired T-cell responses to the HZ/su vaccination for these patients. These results suggest that to ensure satisfactory cellular immunity, it may be better to vaccinate patients with RA with the HZ/su vaccine before the initiation of JAKi treatment.
REFERENCES: [ 1] Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, et al. Risk of Herpes Zoster in Autoimmune and Inflammatory Diseases: Implications for Vaccination. Arthritis Rheumatol. 2016;68(9):2328-37.
[2] Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1843-7.
[3] Cunningham AL, Heineman TC, Lal H, Godeaux O, Chlibek R, Hwang SJ, et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J Infect Dis. 2018;217(11):1750-60.
[4] Källmark H, Bergström T, Nagel J, Gullstrand B, Einarsson JT, Bengtsson AA, Kapetanovic MC. Serologic immunogenicity and safety of herpes zoster subunit vaccine in patients with rheumatoid arthritis receiving Janus kinase inhibitors. Rheumatology (Oxford). 2024;63(7):2024-33.
[5] Levin MJ, Weinberg A. Immune responses to zoster vaccines. Human Vaccines & Immunotherapeutics. 2019;15(4):772-7.
[6] Sieiro-Santos C, Herrero JG, Ordas Martínez J, Álvarez Castro C, López Robles A, Colindres R, et al. Immunogenicity to Herpes Zoster recombinant subunit vaccine in immune-mediated rheumatic patients under treatment with JAK inhibitors. Rheumatology (Oxford). 2024.
Acknowledgements: 1) Meliha C Kapetanovic received support for this study through payments made to the institution by: Unrestricted research grants from Pfizer; Anna-Greta Crafoords Stiftelse för Reumatologisk Forskning; Stiftelsen Konung Gustaf V:s 80-årsfond; Stiftelsen Professor Nanna Svartz Fond; Kockska stiftelser; Södra regionens forskningsstöd; Reumatikerfonden; Greta och Johan Kocks stiftelser; Alfred Österlunds stiftelse; Syskonen Lundgrens stiftelse; SUS donationsfonder; Inger Bendix stiftelse. 2) Hanna Källmark received research funds by Swedish governmental funding of clinical research (ALF).
Disclosure of Interests: Hanna Källmark: None declared, Birgitta Gullstrand: None declared, Fredrik Kahn Research grant from CSL Behring paid to the institution, Elsa Grenmyr: None declared, Jon Thorkell Einarsson: None declared, Anders Bengtsson: None declared, Meliha C Kapetanovic Unrestricted research grants from Pfizer.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (